Abstract
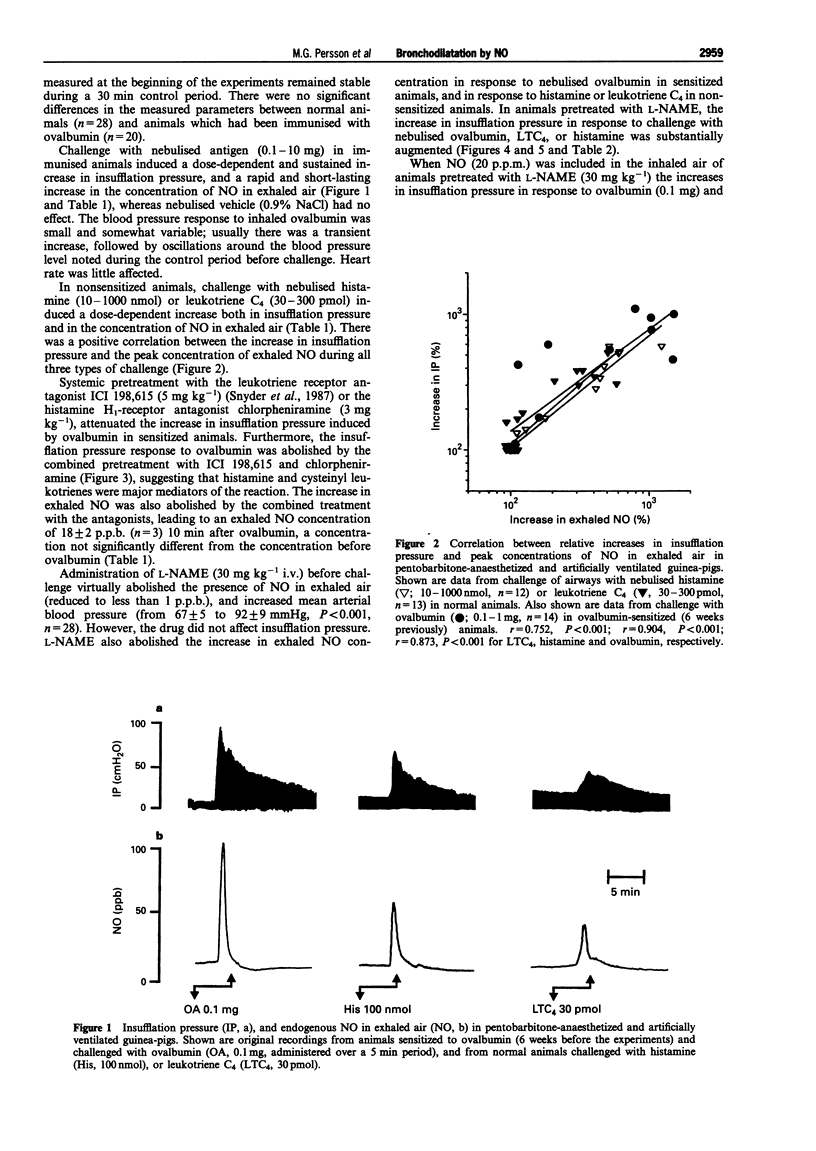
1. The aim of the present study was to investigate the role of nitric oxide (NO), histamine and leukotrienes in bronchial obstruction. For this, guinea-pigs immunised against ovalbumin were studied under anaesthesia during challenge with antigen or agonists. 2. Challenge with nebulised antigen (0.1-1 mg) elicited dose-dependent increases in insufflation pressure which were abolished by combined administration of histamine and leukotriene antagonists. 3. Challenge with nebulised antigen (0.1-1 mg) also elicited dose-dependent increases in the concentration of endogenous nitric oxide in the exhaled air. After an initial peak, exhaled NO concentrations returned to pre-challenge levels. 4. The increase in insufflation pressure and in exhaled NO caused by ovalbumin challenge was inhibited by combined administration of histamine and leukotriene antagonists. 5. In non-immunised guinea-pigs, challenge of the airways with nebulised histamine (10-1000 nmol) or leukotriene C4 (LTC4, 30-300 pmol) elicited dose-dependent increases in insufflation pressure and in concentrations of endogenous NO in exhaled air. 6. The increase in exhaled NO correlated with the increase in insufflation pressure in response to ovalbumin, histamine and LTC4. An inhibitor of endogenous NO synthesis, N omega-nitro-L-arginine methylester (L-NAME, 30 mg kg-1 i.v.) abolished NO exhalation, and markedly augmented the airway responses to ovalbumin, histamine, or LTC4. 7. The potentiation by L-NAME of the increase in insufflation pressure in response to ovalbumin or histamine was prevented by exogenous NO (20 p.p.m.) in the inhaled air. 8. The results indicate that endogenous NO has an inhibitory effect on bronchial obstruction. Increased NO release during allergen challenge is likely to be due to actions of histamine and leukotrienes.
Full text
PDF

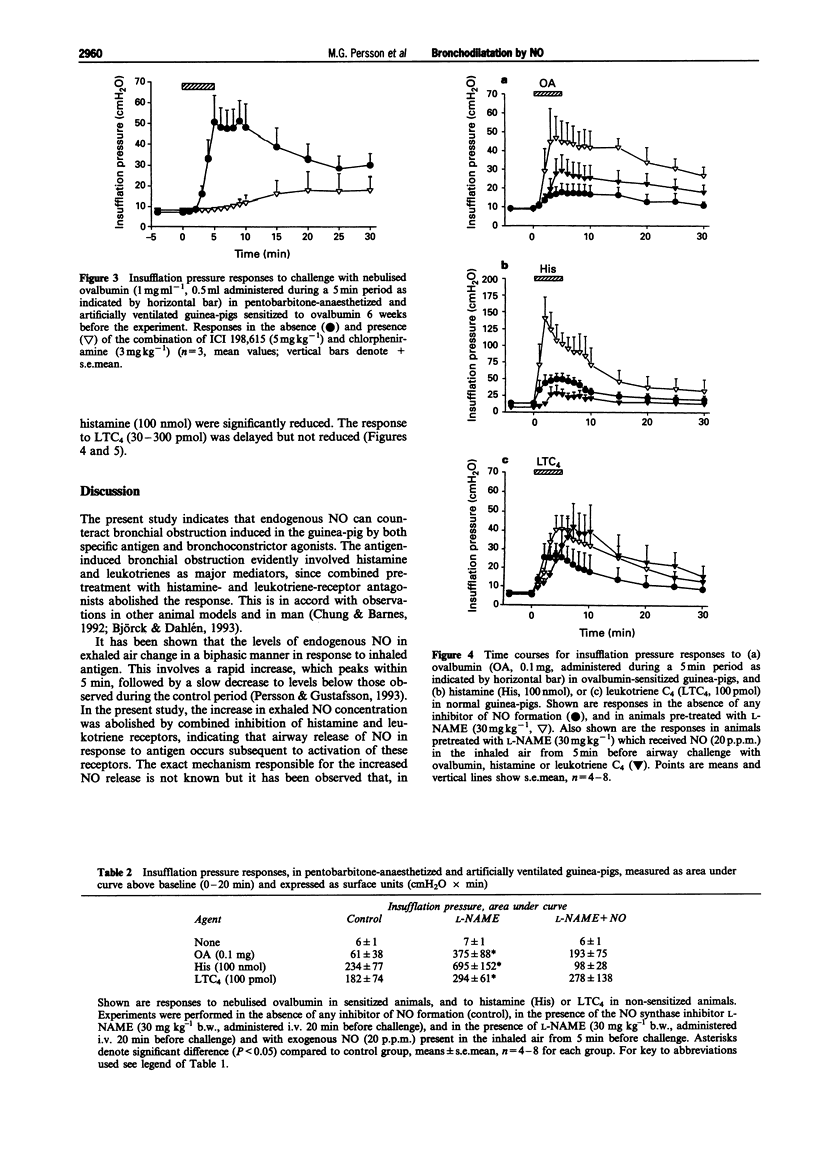
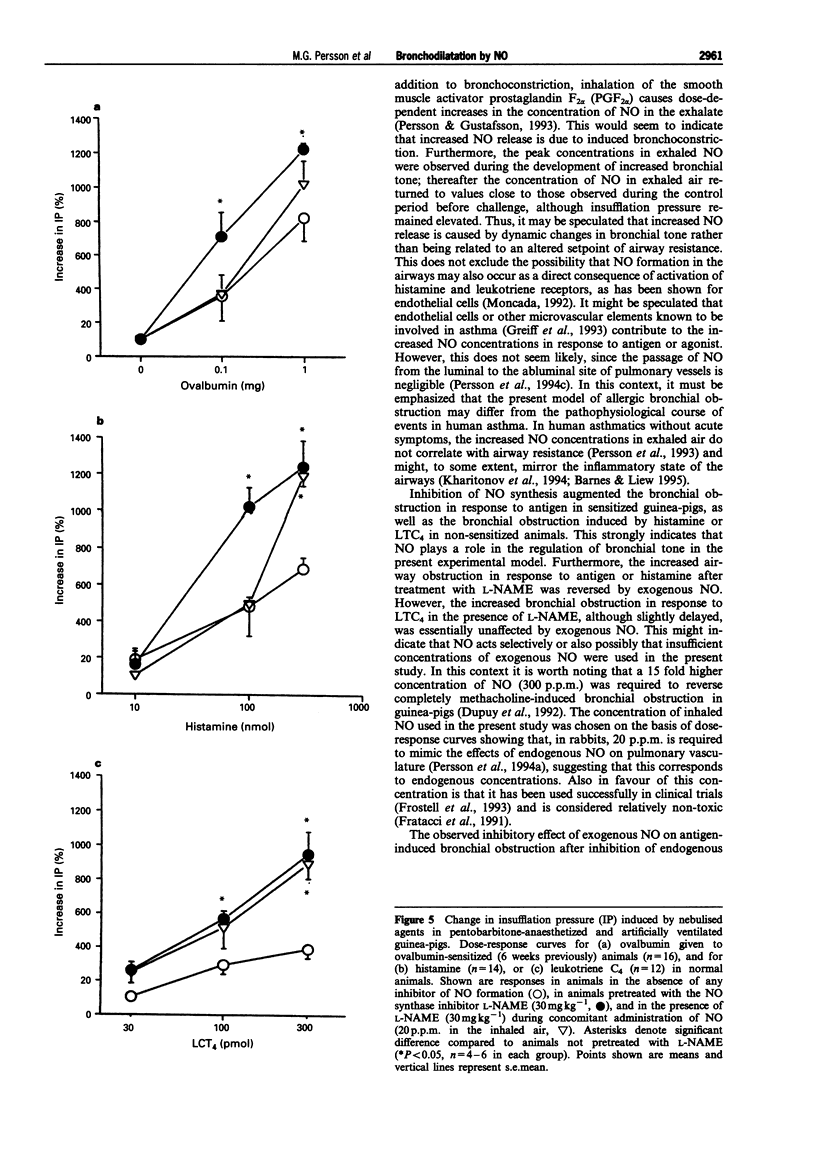

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving K., Weitzberg E., Lundberg J. M. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993 Oct;6(9):1368–1370. [PubMed] [Google Scholar]
- Archer S. L., Tolins J. P., Raij L., Weir E. K. Hypoxic pulmonary vasoconstriction is enhanced by inhibition of the synthesis of an endothelium derived relaxing factor. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1198–1205. doi: 10.1016/0006-291x(89)91796-8. [DOI] [PubMed] [Google Scholar]
- Archer S. Measurement of nitric oxide in biological models. FASEB J. 1993 Feb 1;7(2):349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- Barnes P. J., Liew F. Y. Nitric oxide and asthmatic inflammation. Immunol Today. 1995 Mar;16(3):128–130. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- Belvisi M. G., Stretton C. D., Yacoub M., Barnes P. J. Nitric oxide is the endogenous neurotransmitter of bronchodilator nerves in humans. Eur J Pharmacol. 1992 Jan 14;210(2):221–222. doi: 10.1016/0014-2999(92)90676-u. [DOI] [PubMed] [Google Scholar]
- Björck T., Dahlén S. E. Leukotrienes and histamine mediate IgE-dependent contractions of human bronchi: pharmacological evidence obtained with tissues from asthmatic and non-asthmatic subjects. Pulm Pharmacol. 1993 Mar;6(1):87–96. doi: 10.1006/pulp.1993.1012. [DOI] [PubMed] [Google Scholar]
- Chung K. F., Barnes P. J. Role of inflammatory mediators in asthma. Br Med Bull. 1992 Jan;48(1):135–148. doi: 10.1093/oxfordjournals.bmb.a072530. [DOI] [PubMed] [Google Scholar]
- Dupuy P. M., Shore S. A., Drazen J. M., Frostell C., Hill W. A., Zapol W. M. Bronchodilator action of inhaled nitric oxide in guinea pigs. J Clin Invest. 1992 Aug;90(2):421–428. doi: 10.1172/JCI115877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Mundel P., Mayer B., Preissler U., Philippin B., Kummer W. Nitric oxide synthase in guinea pig lower airway innervation. Neurosci Lett. 1993 Jan 12;149(2):157–160. doi: 10.1016/0304-3940(93)90760-i. [DOI] [PubMed] [Google Scholar]
- Fratacci M. D., Frostell C. G., Chen T. Y., Wain J. C., Jr, Robinson D. R., Zapol W. M. Inhaled nitric oxide. A selective pulmonary vasodilator of heparin-protamine vasoconstriction in sheep. Anesthesiology. 1991 Dec;75(6):990–999. doi: 10.1097/00000542-199112000-00011. [DOI] [PubMed] [Google Scholar]
- Frostell C. G., Blomqvist H., Hedenstierna G., Lundberg J., Zapol W. M. Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology. 1993 Mar;78(3):427–435. doi: 10.1097/00000542-199303000-00005. [DOI] [PubMed] [Google Scholar]
- Gerlach H., Rossaint R., Pappert D., Knorr M., Falke K. J. Autoinhalation of nitric oxide after endogenous synthesis in nasopharynx. Lancet. 1994 Feb 26;343(8896):518–519. doi: 10.1016/s0140-6736(94)91465-6. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. E., Leone A. M., Persson M. G., Wiklund N. P., Moncada S. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991 Dec 16;181(2):852–857. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- Högman M., Frostell C. G., Hedenström H., Hedenstierna G. Inhalation of nitric oxide modulates adult human bronchial tone. Am Rev Respir Dis. 1993 Dec;148(6 Pt 1):1474–1478. doi: 10.1164/ajrccm/148.6_Pt_1.1474. [DOI] [PubMed] [Google Scholar]
- Högman M., Frostell C., Arnberg H., Hedenstierna G. Inhalation of nitric oxide modulates methacholine-induced bronchoconstriction in the rabbit. Eur Respir J. 1993 Feb;6(2):177–180. [PubMed] [Google Scholar]
- Kharitonov S. A., Yates D., Robbins R. A., Logan-Sinclair R., Shinebourne E. A., Barnes P. J. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994 Jan 15;343(8890):133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- Kobzik L., Bredt D. S., Lowenstein C. J., Drazen J., Gaston B., Sugarbaker D., Stamler J. S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993 Oct;9(4):371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Nijkamp F. P., van der Linde H. J., Folkerts G. Nitric oxide synthesis inhibitors induce airway hyperresponsiveness in the guinea pig in vivo and in vitro. Role of the epithelium. Am Rev Respir Dis. 1993 Sep;148(3):727–734. doi: 10.1164/ajrccm/148.3.727. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Gustafsson L. E. Allergen-induced airway obstruction in guinea-pigs is associated with changes in nitric oxide levels in exhaled air. Acta Physiol Scand. 1993 Dec;149(4):461–466. doi: 10.1111/j.1748-1716.1993.tb09643.x. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Gustafsson L. E., Wiklund N. P., Moncada S., Hedqvist P. Endogenous nitric oxide as a probable modulator of pulmonary circulation and hypoxic pressor response in vivo. Acta Physiol Scand. 1990 Dec;140(4):449–457. doi: 10.1111/j.1748-1716.1990.tb09021.x. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Kalzén H., Gustafsson L. E. Oxygen or low concentrations of nitric oxide reverse pulmonary vasoconstriction induced by nitric oxide synthesis inhibition in rabbits. Acta Physiol Scand. 1994 Apr;150(4):405–411. doi: 10.1111/j.1748-1716.1994.tb09705.x. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Midtvedt T., Leone A. M., Gustafsson L. E. Ca(2+)-dependent and Ca(2+)-independent exhaled nitric oxide, presence in germ-free animals, and inhibition by arginine analogues. Eur J Pharmacol. 1994 Oct 13;264(1):13–20. doi: 10.1016/0014-2999(94)90629-7. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Zetterström O., Agrenius V., Ihre E., Gustafsson L. E. Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994 Jan 15;343(8890):146–147. doi: 10.1016/s0140-6736(94)90935-0. [DOI] [PubMed] [Google Scholar]
- Snyder D. W., Giles R. E., Keith R. A., Yee Y. K., Krell R. D. In vitro pharmacology of ICI 198,615: a novel, potent and selective peptide leukotriene antagonist. J Pharmacol Exp Ther. 1987 Nov;243(2):548–556. [PubMed] [Google Scholar]
- Sprague R. S., Thiemermann C., Vane J. R. Endogenous endothelium-derived relaxing factor opposes hypoxic pulmonary vasoconstriction and supports blood flow to hypoxic alveoli in anesthetized rabbits. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8711–8715. doi: 10.1073/pnas.89.18.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]


