Abstract
1. To examine whether cytosolic Ca2+ in smooth muscle cells distributes evenly, cytosolic Ca2+ levels were measured with two different Ca2+ indicators in the ferret isolated portal vein; a fluorescent indicator, fura-PE3, that shows the average Ca2+ level, and a photoprotein, aequorin, that preferentially shows a high Ca2+ compartment. 2. A noradrenaline (10 microM)-induced sustained contraction was associated with a sustained increase in the fura-PE3 signal, or a transient increase followed by small sustained increase in the aequorin signal. A high K(+)-induced contraction was associated with a sustained increase in both the fura-PE3 and aequorin signals. 3. A second application of noradrenaline or high K+ induced reproducible contractions and fura-PE3 signals. In contrast, the aequorin signal resulting from a second application of noradrenaline or high K+ was much smaller than the first signal. 4. Following a 13 h but not a 3 h resting period, the aequorin signal stimulated by noradrenaline or high K+ recovered, without any change in the contractile response. 5. In Ca(2+)-free solution, high K+ was ineffective, whereas noradrenaline induced only a small aequorin signal and contraction compared to those obtained in the presence of external Ca2+. After the addition of Ca2+, the first application of noradrenaline induced a large aequorin signal and a large contraction, although a second application induced a much smaller aequorin signal accompanied by a large contraction. 6. These results suggest that high K+ and noradrenaline increase Ca2+ in at least two cytosolic compartments; a compartment that is coupled to the contractile mechanism ('contractile' Ca2+ compartment; major portion of cytoplasm containing contractile elements) and a compartment that is not coupled to contractile mechanisms ('non-contractile' Ca2+ compartment; small sub-membrane area that does not contain contractile elements). On stimulation, the Ca2+ level in the 'contractile' compartment may increase to a level high enough to stimulate myosin light chain kinase but not so high as to consume aequorin rapidly. In contrast, the Ca2+ level in the 'non-contractile' compartment may increase so greatly that aequorin in this compartment is rapidly consumed. These two compartments may be separated by a diffusion barrier and, during a resting period, aequorin may slowly diffuse from the 'contractile' compartment to the 'non-contractile' compartment and thus restore the full aequorin signal. An increase in Ca2+ in the 'non-contractile' compartment seems to be dependent mainly on Ca2+ influx and partly on Ca2+ release.
Full text
PDF
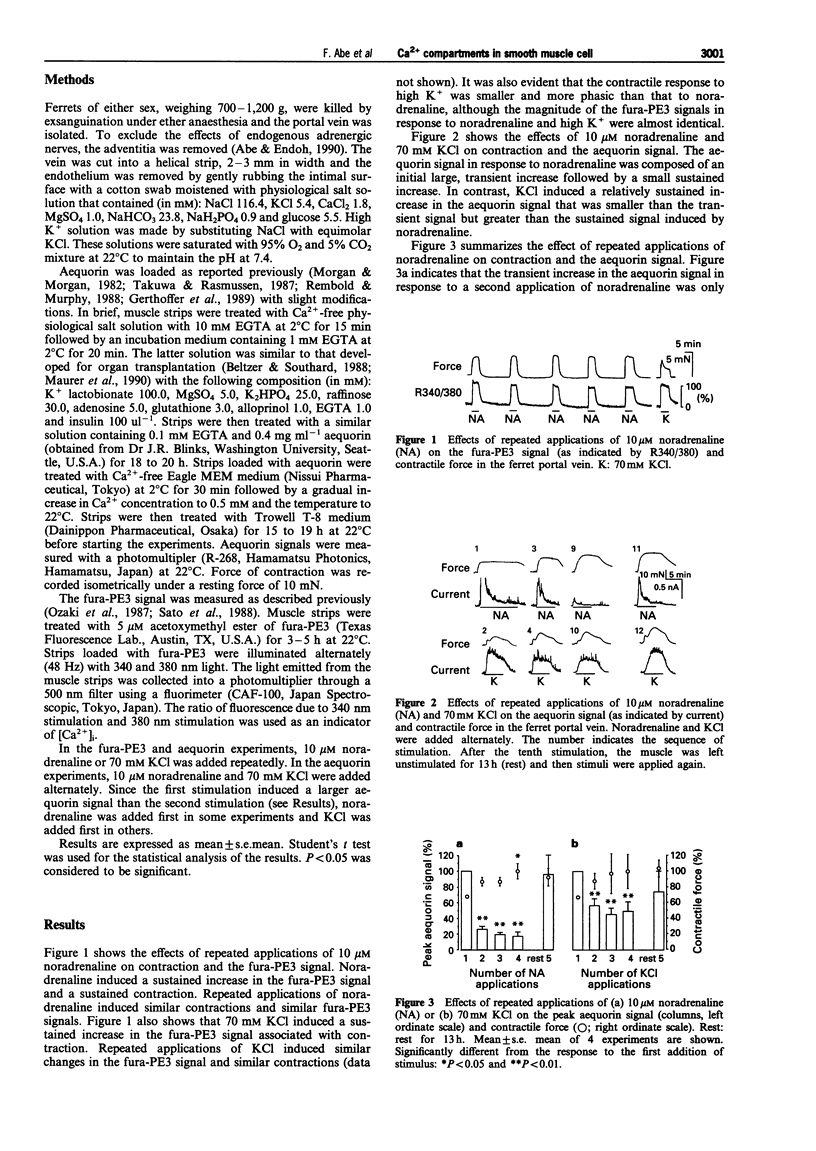


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belzer F. O., Southard J. H. Principles of solid-organ preservation by cold storage. Transplantation. 1988 Apr;45(4):673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- Chen Q., van Breemen C. The superficial buffer barrier in venous smooth muscle: sarcoplasmic reticulum refilling and unloading. Br J Pharmacol. 1993 Jun;109(2):336–343. doi: 10.1111/j.1476-5381.1993.tb13575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter E. F., Kuhn M. A., Fay F. S. Detection of changes in near-membrane Ca2+ concentration using a novel membrane-associated Ca2+ indicator. J Biol Chem. 1994 Apr 1;269(13):10141–10149. [PubMed] [Google Scholar]
- Gerthoffer W. T., Murphey K. A., Gunst S. J. Aequorin luminescence, myosin phosphorylation, and active stress in tracheal smooth muscle. Am J Physiol. 1989 Dec;257(6 Pt 1):C1062–C1068. doi: 10.1152/ajpcell.1989.257.6.C1062. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Parker I. Calcium transients evoked by electrical stimulation of smooth muscle from guinea-pig ileum recorded by the use of Fura-2. J Physiol. 1988 Dec;407:117–134. doi: 10.1113/jphysiol.1988.sp017406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989 Aug;10(8):320–325. doi: 10.1016/0165-6147(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Karaki H., Kubota H., Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979 Jun 15;56(3):237–245. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- Karaki H., Sato K., Ozaki H. Different effects of verapamil on cytosolic Ca2+ and contraction in norepinephrine-stimulated vascular smooth muscle. Jpn J Pharmacol. 1991 Jan;55(1):35–42. doi: 10.1254/jjp.55.35. [DOI] [PubMed] [Google Scholar]
- Karaki H., Weiss G. B. Calcium release in smooth muscle. Life Sci. 1988;42(2):111–122. doi: 10.1016/0024-3205(88)90674-1. [DOI] [PubMed] [Google Scholar]
- Kargacin G. J. Calcium signaling in restricted diffusion spaces. Biophys J. 1994 Jul;67(1):262–272. doi: 10.1016/S0006-3495(94)80477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S., Ozaki H., Karaki H. The effects of ATP and alpha,beta-methylene-ATP on cytosolic Ca2+ level and force in rat isolated aorta. Br J Pharmacol. 1993 Sep;110(1):263–268. doi: 10.1111/j.1476-5381.1993.tb13803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer E. J., Swanson D. K., DeBoer L. W. Comparison of UW and Collins solution for preservation of the rat heart. Transplant Proc. 1990 Apr;22(2):548–550. [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Sato K., Satoh T., Karaki H. Simultaneous recordings of calcium signals and mechanical activity using fluorescent dye fura 2 in isolated strips of vascular smooth muscle. Jpn J Pharmacol. 1987 Nov;45(3):429–433. doi: 10.1254/jjp.45.429. [DOI] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res. 1988 Sep;63(3):593–603. doi: 10.1161/01.res.63.3.593. [DOI] [PubMed] [Google Scholar]
- Sakata K., Ozaki H., Kwon S. C., Karaki H. Effects of endothelin on the mechanical activity and cytosolic calcium level of various types of smooth muscle. Br J Pharmacol. 1989 Oct;98(2):483–492. doi: 10.1111/j.1476-5381.1989.tb12621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Regeneration of the photoprotein aequorin. Nature. 1975 Jul 17;256(5514):236–238. doi: 10.1038/256236a0. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Rasmussen H. Measurement of cytoplasmic free Ca2+ concentration in rabbit aorta using the photoprotein, aequorin. Effect of atrial natriuretic peptide on agonist-induced Ca2+ signal generation. J Clin Invest. 1987 Jul;80(1):248–257. doi: 10.1172/JCI113055. [DOI] [PMC free article] [PubMed] [Google Scholar]


