Abstract
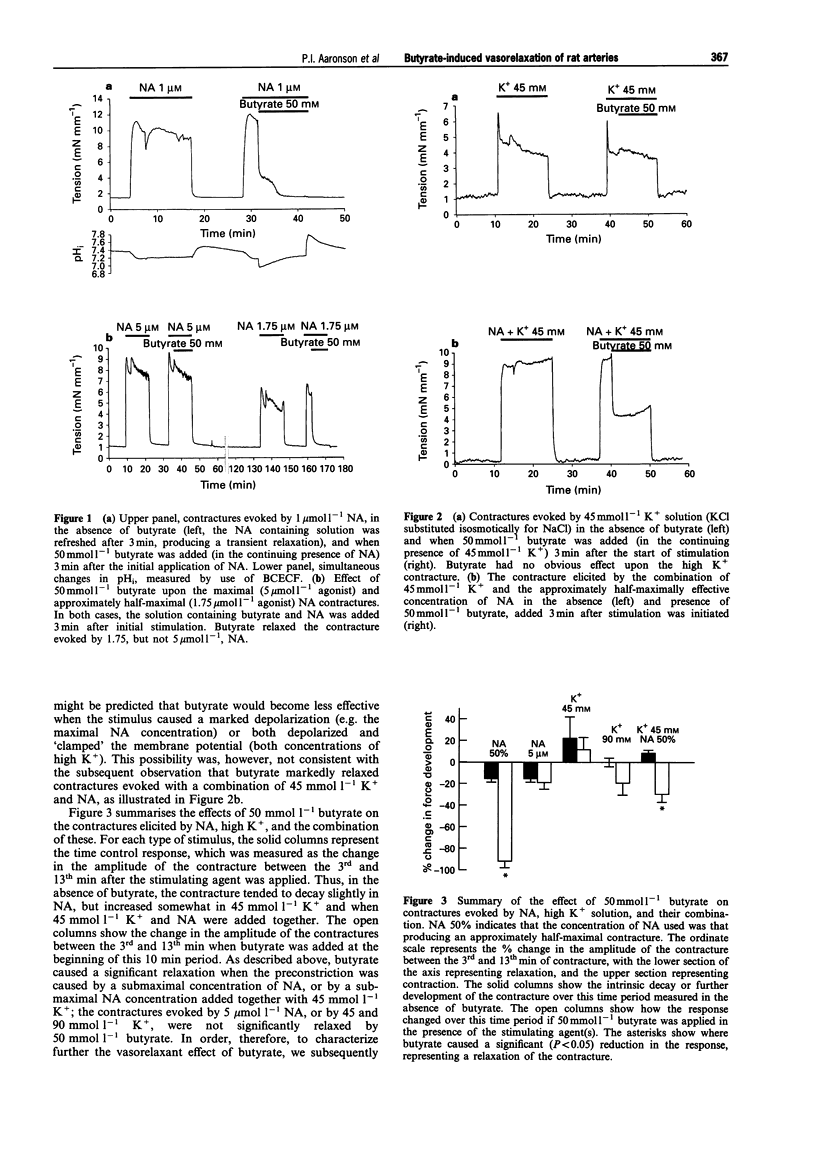
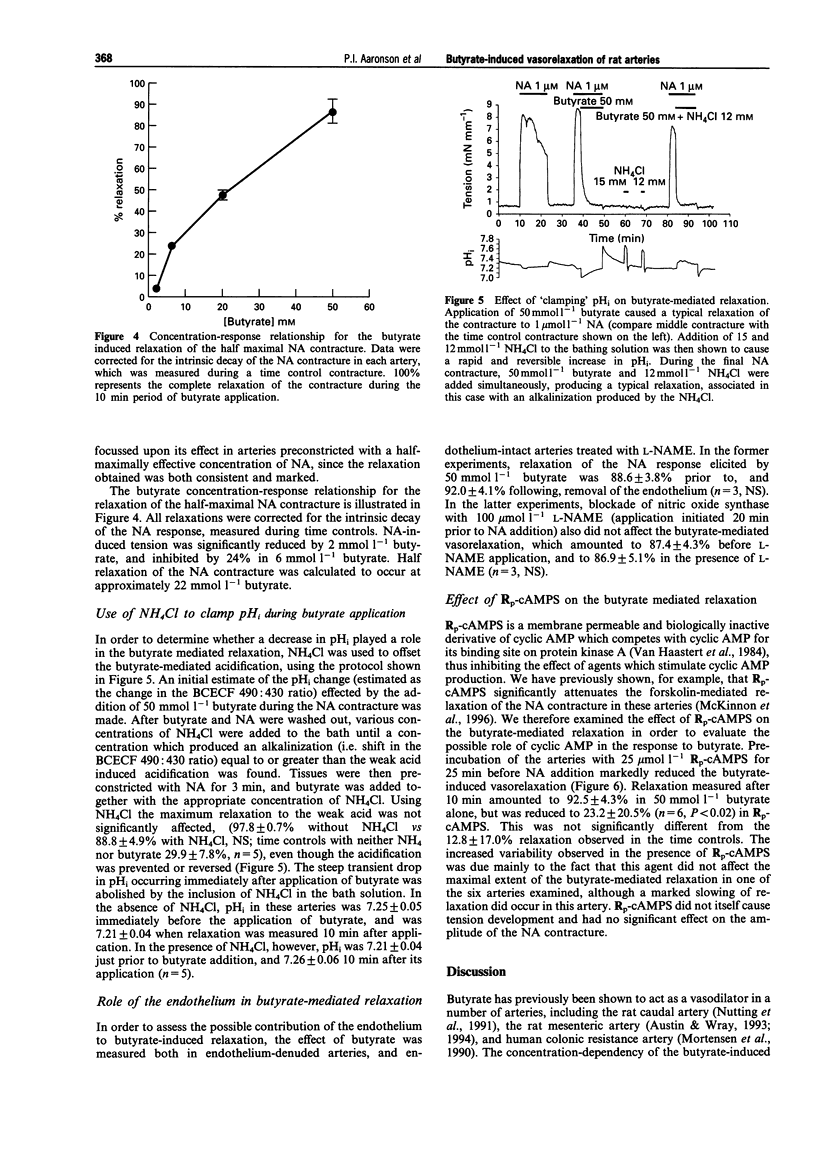
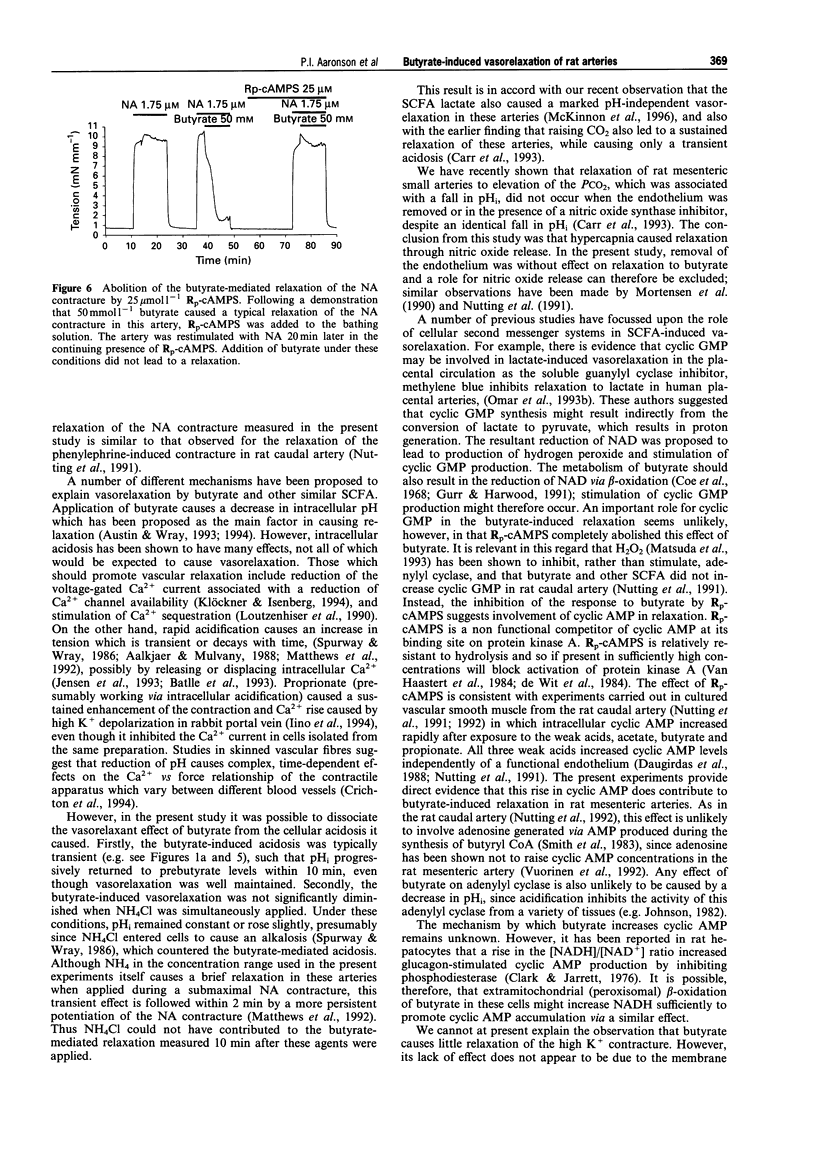
1. The vasorelaxant effect of the sodium salt of the short chain fatty acid, butyrate, on preconstricted rat small mesenteric arteries (mean inner diameter approximately 300 microns) was characterized. Isometric force development was measured with a myograph, and intracellular pH (pHi) was simultaneously monitored, in arteries loaded with the fluorescent dye BCECF in its acetomethoxy form. Sodium butyrate (substituted isosmotically for NaCl) was applied to arteries after noradrenaline (NA) or high K+ contractures were established. 2. Arteries preconstricted with a concentration of NA inducing an approximately half maximal contraction were relaxed by 91.5 +/- 6.3% by 50 mmol l-1 butyrate. This concentration of butyrate did not, however, cause a significant relaxation of contractures to a maximal (5 mumol l-1) NA concentration, and also failed to relax significantly contractures stimulated by high (45 and 90 mmol l-1) K+ solutions. Contractures elicited with a combination of NA (at a submaximal concentration) and 45 mmol l-1 K+ were, however, markedly relaxed by butyrate. 3. Investigation of the concentration-dependency of the butyrate-induced relaxation of the half maximal NA response revealed an EC50 for butyrate of approximately 22 mmol l-1. 4. Sodium butyrate (50 mmol l-1) caused pHi to decrease from 7.25 +/- 0.02 to 6.89 +/- 0.08 (n = 4, P < 0.001). However, the vasorelaxant effect of butyrate on the submaximal NA contracture was not significantly modified when this fall in intracellular pH was prevented by the simultaneous application of NH4Cl. 5. Butyrate-induced relaxation was also unaffected by endothelial denudation and inhibition of NO synthase with N omega-nitro-L-arginine methyl ester (100 mumol l-1). 6. The relaxation of the NA contracture by 50 mmol l-1 sodium butyrate was abolished in arteries pretreated with the cyclic AMP antagonist Rp-cAMPS (25 mumol l-1). 7. We conclude that the butyrate-induced relaxation of the NA contracture is independent of intracellular acidification. The ability of Rp-cAMPS to abolish the butyrate relaxation indicates that stimulation of the cyclic AMP second messenger system may play an important role in mediating this effect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Mulvany M. J. Effect of changes in intracellular pH on the contractility of rat resistance vessels. Prog Biochem Pharmacol. 1988;23:150–158. [PubMed] [Google Scholar]
- Austin C., Wray S. A quantitative study of the relation between intracellular pH and force in rat mesenteric vascular smooth muscle. Pflugers Arch. 1994 Jun;427(3-4):270–276. doi: 10.1007/BF00374534. [DOI] [PubMed] [Google Scholar]
- Austin C., Wray S. Extracellular pH signals affect rat vascular tone by rapid transduction into intracellular pH changes. J Physiol. 1993 Jul;466:1–8. [PMC free article] [PubMed] [Google Scholar]
- Batlle D. C., Peces R., LaPointe M. S., Ye M., Daugirdas J. T. Cytosolic free calcium regulation in response to acute changes in intracellular pH in vascular smooth muscle. Am J Physiol. 1993 Apr;264(4 Pt 1):C932–C943. doi: 10.1152/ajpcell.1993.264.4.C932. [DOI] [PubMed] [Google Scholar]
- Carr P., Graves J. E., Poston L. Carbon dioxide induced vasorelaxation in rat mesenteric small arteries precontracted with noradrenaline is endothelium dependent and mediated by nitric oxide. Pflugers Arch. 1993 May;423(3-4):343–345. doi: 10.1007/BF00374415. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Jarrett I. G. Responsiveness to glucagon by isolated rat hepatocytes controlled by the redox state of the cytosolic nicotinamide--adenine dinucleotide couple acting on adenosine 3':5'-cyclic monophosphate phosphodiesterase. Biochem J. 1978 Dec 15;176(3):805–816. doi: 10.1042/bj1760805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe J., Detar R., Bohr D. F. Substrates and vascular smooth muscle contraction. Am J Physiol. 1968 Feb;214(2):245–250. doi: 10.1152/ajplegacy.1968.214.2.245. [DOI] [PubMed] [Google Scholar]
- Crichton C. A., Templeton A. G., Smith G. L. Effect of altered bathing pH on calcium activated force in alpha toxin permeabilised rat portal vein and human umbilical artery. Cardiovasc Res. 1994 Sep;28(9):1378–1384. doi: 10.1093/cvr/28.9.1378. [DOI] [PubMed] [Google Scholar]
- Daugirdas J. T., Swanson V., Islam S., Nutting C., Kim D. D., Wang X. A., Fiscus R. R. Acetate causes endothelium-independent increases in cyclic AMP in rat caudal artery. Am J Physiol. 1988 Dec;255(6 Pt 2):H1378–H1383. doi: 10.1152/ajpheart.1988.255.6.H1378. [DOI] [PubMed] [Google Scholar]
- Graves J., Poston L. Beta-adrenoceptor agonist mediated relaxation of rat isolated resistance arteries: a role for the endothelium and nitric oxide. Br J Pharmacol. 1993 Mar;108(3):631–637. doi: 10.1111/j.1476-5381.1993.tb12853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino S., Hayashi H., Saito H., Tokuno H., Tomita T. Effects of intracellular pH on calcium currents and intracellular calcium ions in the smooth muscle of rabbit portal vein. Exp Physiol. 1994 Sep;79(5):669–680. doi: 10.1113/expphysiol.1994.sp003799. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Hughes A., Boonen H. C., Aalkjaer C. Force, membrane potential, and [Ca2+]i during activation of rat mesenteric small arteries with norepinephrine, potassium, aluminum fluoride, and phorbol ester. Effects of changes in pHi. Circ Res. 1993 Aug;73(2):314–324. doi: 10.1161/01.res.73.2.314. [DOI] [PubMed] [Google Scholar]
- Johnson R. A. Changes in pH sensitivity of adenylate cyclase specifically induced by fluoride and vanadate. Arch Biochem Biophys. 1982 Oct 1;218(1):68–76. doi: 10.1016/0003-9861(82)90322-8. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Intracellular pH modulates the availability of vascular L-type Ca2+ channels. J Gen Physiol. 1994 Apr;103(4):647–663. doi: 10.1085/jgp.103.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutzenhiser R., Matsumoto Y., Okawa W., Epstein M. H(+)-induced vasodilation of rat aorta is mediated by alterations in intracellular calcium sequestration. Circ Res. 1990 Aug;67(2):426–439. doi: 10.1161/01.res.67.2.426. [DOI] [PubMed] [Google Scholar]
- Masuda H., Kaneko M., Hong R. B., Ikegaya T., Hayashi H., Kobayashi A., Yamazaki N. Effects of hydrogen peroxide on stimulatory guanine nucleotide-binding protein in rat heart. Jpn Circ J. 1993 Oct;57(10):1007–1015. doi: 10.1253/jcj.57.1007. [DOI] [PubMed] [Google Scholar]
- Matthews J. G., Graves J. E., Poston L. Relationships between pHi and tension in isolated rat mesenteric resistance arteries. J Vasc Res. 1992 Jul-Aug;29(4):330–340. doi: 10.1159/000158948. [DOI] [PubMed] [Google Scholar]
- Mortensen F. V., Nielsen H., Mulvany M. J., Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut. 1990 Dec;31(12):1391–1394. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nutting C. W., Islam S., Daugirdas J. T. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am J Physiol. 1991 Aug;261(2 Pt 2):H561–H567. doi: 10.1152/ajpheart.1991.261.2.H561. [DOI] [PubMed] [Google Scholar]
- Nutting C. W., Islam S., Ye M. H., Batlle D. C., Daugirdas J. T. The vasorelaxant effects of acetate: role of adenosine, glycolysis, lyotropism, and pHi and Cai2+. Kidney Int. 1992 Jan;41(1):166–174. doi: 10.1038/ki.1992.23. [DOI] [PubMed] [Google Scholar]
- Okada K., Tsai P., Briner V. A., Caramelo C., Schrier R. W. Effects of extra- and intracellular pH on vascular action of arginine vasopressin. Am J Physiol. 1991 Jan;260(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1991.260.1.F39. [DOI] [PubMed] [Google Scholar]
- Omar H. A., Figueroa R., Tejani N., Wolin M. S. Properties of a lactate-induced relaxation in human placental arteries and veins. Am J Obstet Gynecol. 1993 Oct;169(4):912–918. doi: 10.1016/0002-9378(93)90026-f. [DOI] [PubMed] [Google Scholar]
- Spurway N. C., Wray S. A phosphorus nuclear magnetic resonance study of metabolites and intracellular pH in rabbit vascular smooth muscle. J Physiol. 1987 Dec;393:57–71. doi: 10.1113/jphysiol.1987.sp016810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J., Van Driel R., Jastorff B., Baraniak J., Stec W. J., De Wit R. J. Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem. 1984 Aug 25;259(16):10020–10024. [PubMed] [Google Scholar]
- Vuorinen P., Pörsti I., Metsä-Ketelä T., Manninen V., Vapaatalo H., Laustiola K. E. Endothelium-dependent and -independent effects of exogenous ATP, adenosine, GTP and guanosine on vascular tone and cyclic nucleotide accumulation of rat mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):279–284. doi: 10.1111/j.1476-5381.1992.tb14246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit R. J., Hekstra D., Jastorff B., Stec W. J., Baraniak J., Van Driel R., Van Haastert P. J. Inhibitory action of certain cyclophosphate derivatives of cAMP on cAMP-dependent protein kinases. Eur J Biochem. 1984 Jul 16;142(2):255–260. doi: 10.1111/j.1432-1033.1984.tb08279.x. [DOI] [PubMed] [Google Scholar]


