Abstract
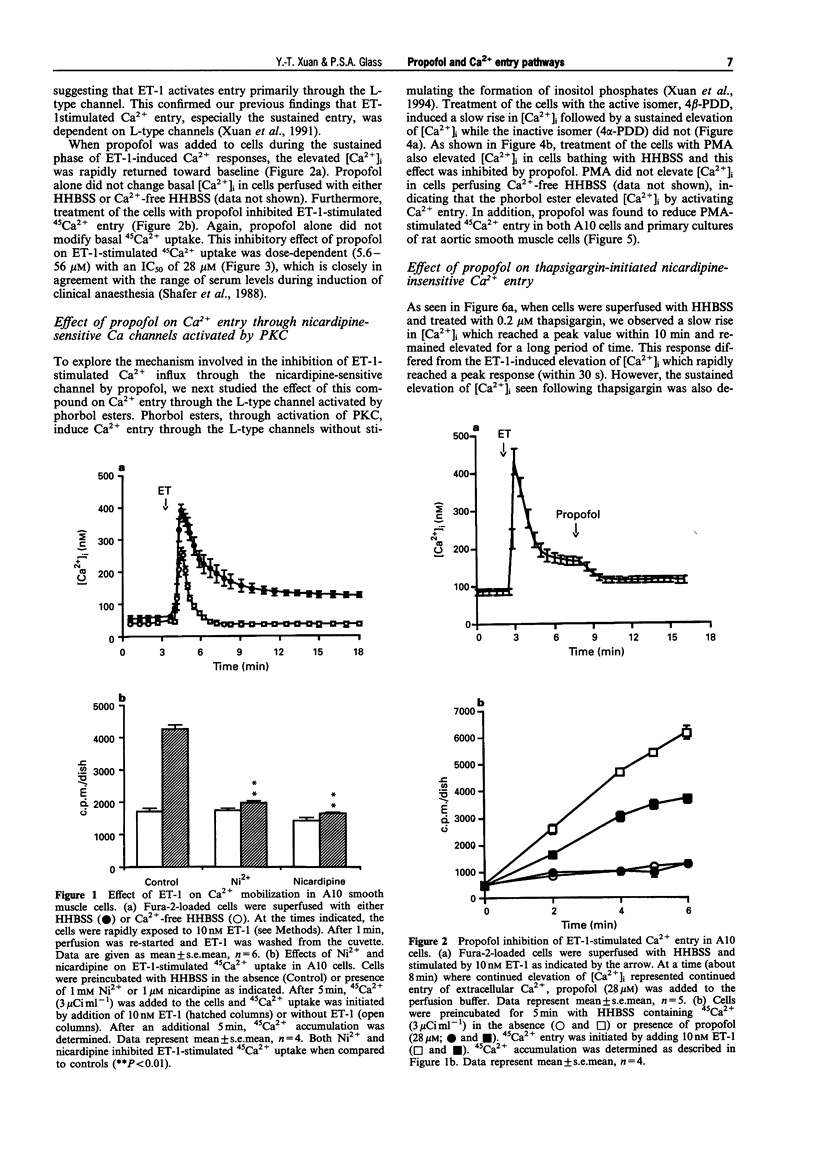
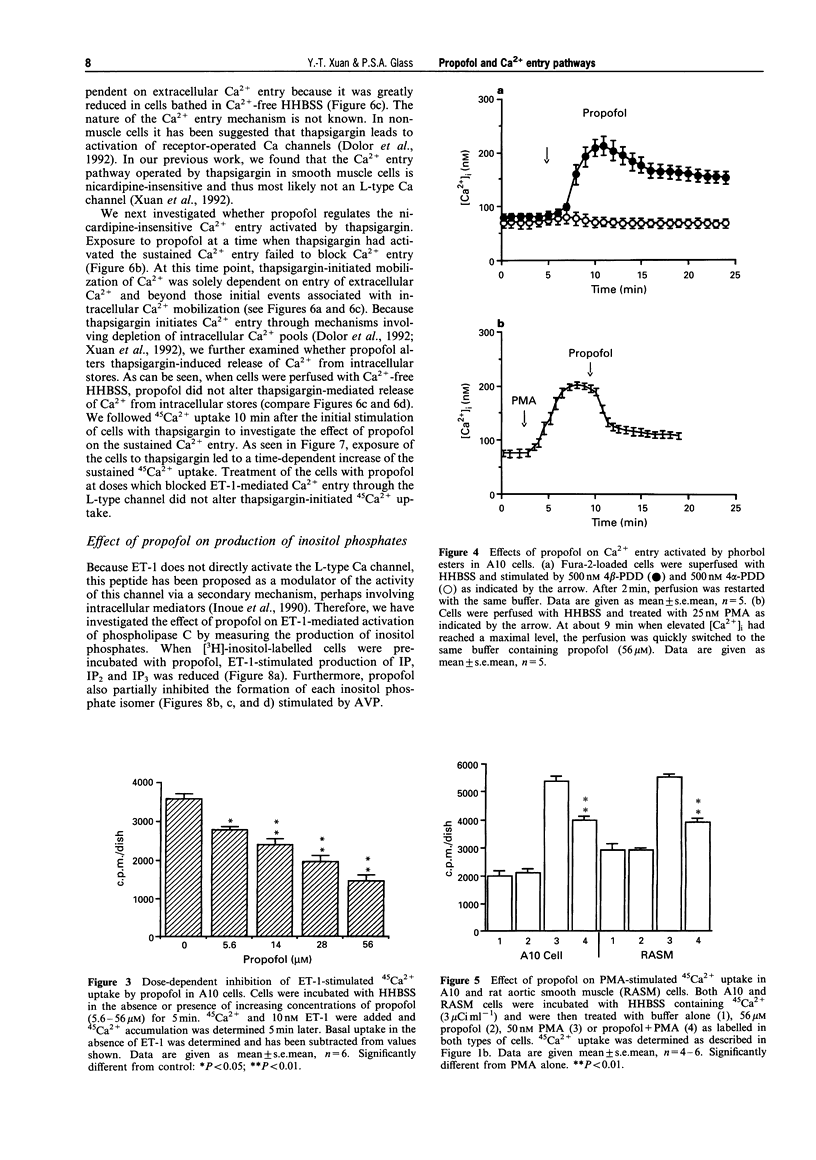
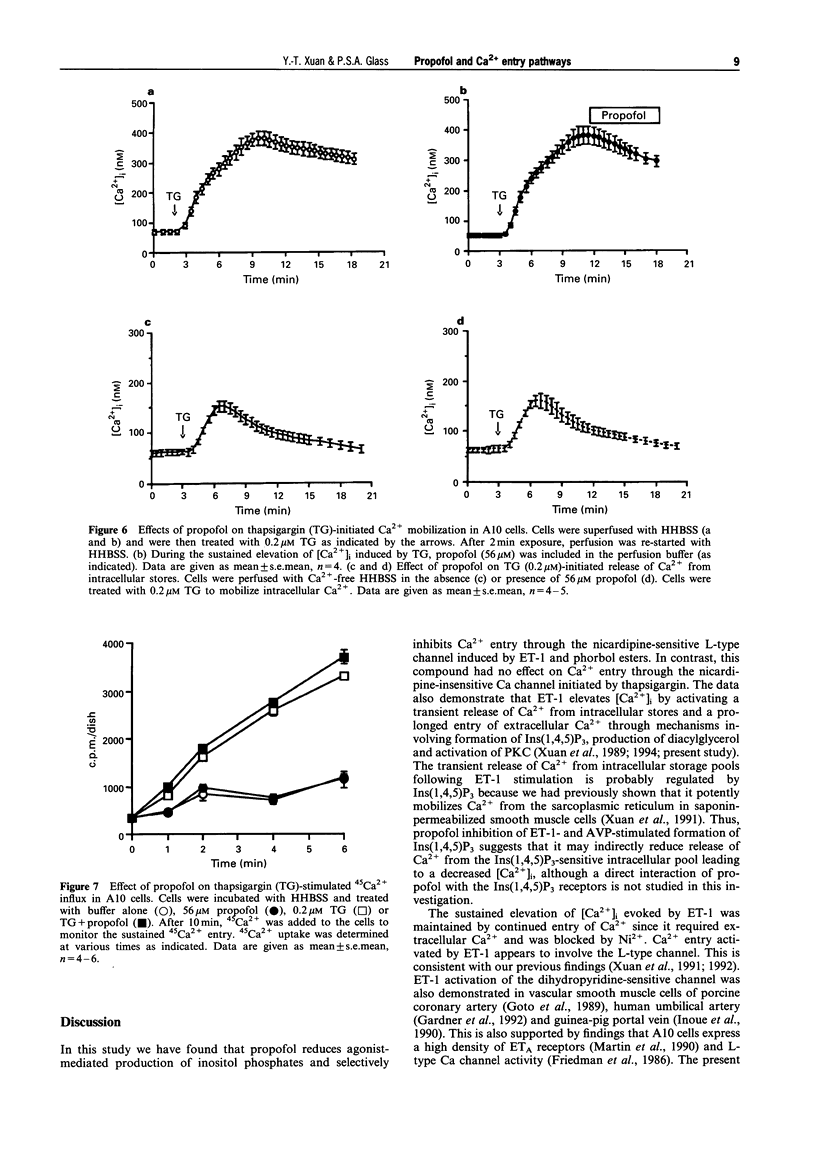
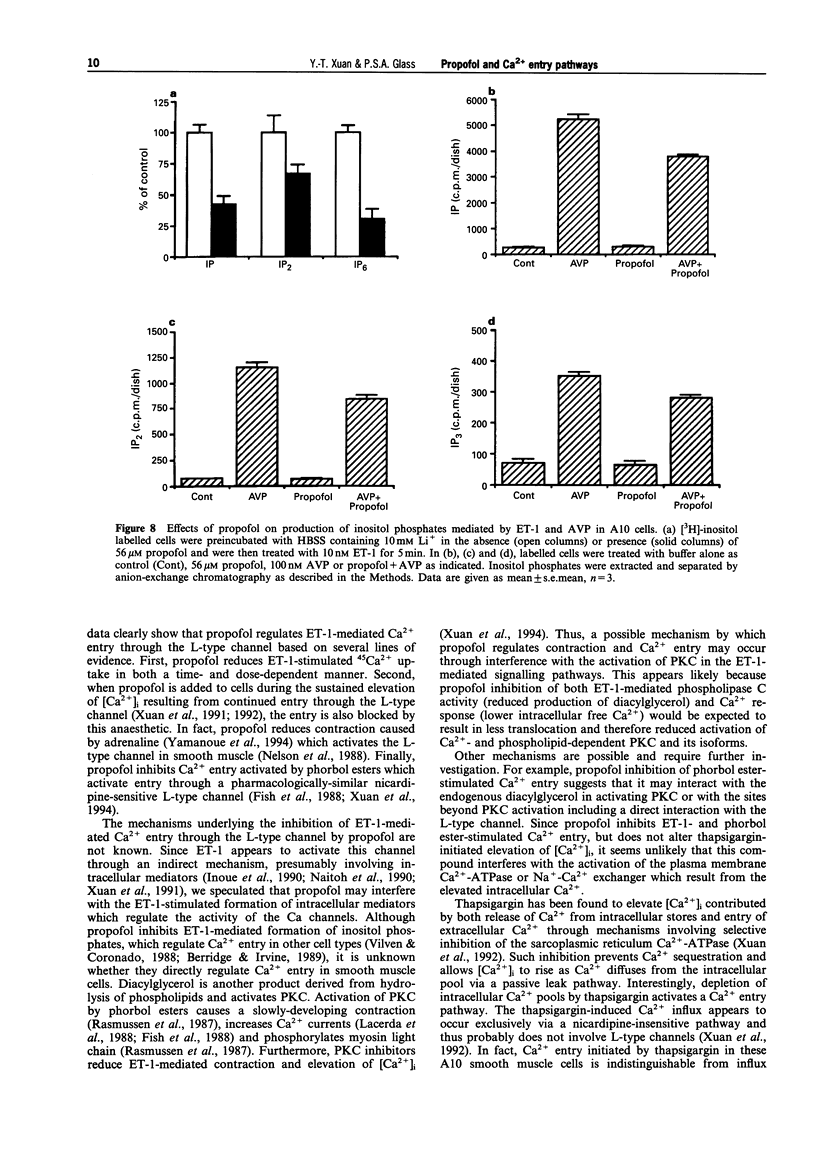
1. We have investigated the effect of propofol, an intravenous anaesthetic, on the intracellular calcium concentration ([Ca2+]i), Ca2+ entry pathways and on inositol phosphate formation in vascular smooth muscle cells. [Ca2+]i and Ca2+ flux were monitored with the Ca(2+)-sensitive fluorescent dye, fura-2, and by 45Ca2+ uptake. Production of labelled inositol phosphates was analysed by anion-exchange chromatography. 2. Treatment of the cells with endothelin-1 (ET-1) increased formation of inositol phosphates and elevated [Ca2+]i due to both release of Ca2+ from intracellular pools and prolonged entry of Ca2+ from outside the cell. Propofol reduced production of inositol phosphates mediated by ET-1 and arginine vasopressin which activate phospholipase C. 3. The sustained Ca2+ entry stimulated by ET-1 was found to occur through the activation of L-type Ca channels. This was inhibited by propofol in a dose-dependent manner. 4. Activation of protein kinase C (PKC) by phorbol esters activated a pharmacologically-similar channel and produced a similar change in [Ca2+]i due to Ca2+ entry. The entry was blocked by an L-type channel antagonist, nicardipine and by the anaesthetic drug, propofol. 5. Treatment of the cells with thapsigargin, a selective inhibitor of the sarcoplasmic reticulum Ca(2+)-ATPase, also elevated [Ca2+]i by inducing the release of intracellular Ca2+ and the continued entry of extracellular Ca2+ through a nicardipine-insensitive Ca channel. Neither release nor entry induced by thapsigargin was affected by propofol. 6. These findings suggest that propofol selectively inhibits Ca2+ entry through the L-type channel induced by ET-1 and phorbol esters but has no effects on Ca2+ entry via the nicardipine-insensitive channel and on Ca2+ release from intracellular pools initiated by thapsigargin. This may represent one of the mechanisms responsible for propofol-induced vasodilatation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley G. N., Gent J. P., Goodchild C. S. Vascular effects of propofol: smooth muscle relaxation in isolated veins and arteries. J Pharm Pharmacol. 1989 Nov;41(11):797–798. doi: 10.1111/j.2042-7158.1989.tb06371.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Brüssel T., Theissen J. L., Vigfusson G., Lunkenheimer P. P., Van Aken H., Lawin P. Hemodynamic and cardiodynamic effects of propofol and etomidate: negative inotropic properties of propofol. Anesth Analg. 1989 Jul;69(1):35–40. [PubMed] [Google Scholar]
- Coates D. P., Monk C. R., Prys-Roberts C., Turtle M. Hemodynamic effects of infusions of the emulsion formulation of propofol during nitrous oxide anesthesia in humans. Anesth Analg. 1987 Jan;66(1):64–70. [PubMed] [Google Scholar]
- Cook D. J., Housmans P. R. Mechanism of the negative inotropic effect of propofol in isolated ferret ventricular myocardium. Anesthesiology. 1994 Apr;80(4):859–871. doi: 10.1097/00000542-199404000-00020. [DOI] [PubMed] [Google Scholar]
- Dolor R. J., Hurwitz L. M., Mirza Z., Strauss H. C., Whorton A. R. Regulation of extracellular calcium entry in endothelial cells: role of intracellular calcium pool. Am J Physiol. 1992 Jan;262(1 Pt 1):C171–C181. doi: 10.1152/ajpcell.1992.262.1.C171. [DOI] [PubMed] [Google Scholar]
- Fish R. D., Sperti G., Colucci W. S., Clapham D. E. Phorbol ester increases the dihydropyridine-sensitive calcium conductance in a vascular smooth muscle cell line. Circ Res. 1988 May;62(5):1049–1054. doi: 10.1161/01.res.62.5.1049. [DOI] [PubMed] [Google Scholar]
- Friedman M. E., Suarez-Kurtz G., Kaczorowski G. J., Katz G. M., Reuben J. P. Two calcium currents in a smooth muscle cell line. Am J Physiol. 1986 Apr;250(4 Pt 2):H699–H703. doi: 10.1152/ajpheart.1986.250.4.H699. [DOI] [PubMed] [Google Scholar]
- Gardner J. P., Tokudome G., Tomonari H., Maher E., Hollander D., Aviv A. Endothelin-induced calcium responses in human vascular smooth muscle cells. Am J Physiol. 1992 Jan;262(1 Pt 1):C148–C155. doi: 10.1152/ajpcell.1992.262.1.C148. [DOI] [PubMed] [Google Scholar]
- Goto K., Kasuya Y., Matsuki N., Takuwa Y., Kurihara H., Ishikawa T., Kimura S., Yanagisawa M., Masaki T. Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc Natl Acad Sci U S A. 1989 May;86(10):3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Inoue Y., Oike M., Nakao K., Kitamura K., Kuriyama H. Endothelin augments unitary calcium channel currents on the smooth muscle cell membrane of guinea-pig portal vein. J Physiol. 1990 Apr;423:171–191. doi: 10.1113/jphysiol.1990.sp018017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Martin E. R., Brenner B. M., Ballermann B. J. Heterogeneity of cell surface endothelin receptors. J Biol Chem. 1990 Aug 15;265(23):14044–14049. [PubMed] [Google Scholar]
- Naitoh T., Toyo-Oka T., Sugimoto T. An endogenous Ca2+ channel agonist, endothelin-1, does not directly activate partially purified dihydropyridine-sensitive Ca2+ channel from cardiac muscle in a reconstituted system. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1205–1210. doi: 10.1016/0006-291x(90)90813-3. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Park W. K., Lynch C., 3rd, Johns R. A. Effects of propofol and thiopental in isolated rat aorta and pulmonary artery. Anesthesiology. 1992 Nov;77(5):956–963. doi: 10.1097/00000542-199211000-00019. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Takuwa Y., Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987 Sep;1(3):177–185. [PubMed] [Google Scholar]
- Rosenthal W., Hescheler J., Trautwein W., Schultz G. Control of voltage-dependent Ca2+ channels by G protein-coupled receptors. FASEB J. 1988 Sep;2(12):2784–2790. doi: 10.1096/fasebj.2.12.2457531. [DOI] [PubMed] [Google Scholar]
- Rouby J. J., Andreev A., Léger P., Arthaud M., Landault C., Vicaut E., Maistre G., Eurin J., Gandjbakch I., Viars P. Peripheral vascular effects of thiopental and propofol in humans with artificial hearts. Anesthesiology. 1991 Jul;75(1):32–42. doi: 10.1097/00000542-199107000-00007. [DOI] [PubMed] [Google Scholar]
- Sebel P. S., Lowdon J. D. Propofol: a new intravenous anesthetic. Anesthesiology. 1989 Aug;71(2):260–277. [PubMed] [Google Scholar]
- Shafer A., Doze V. A., Shafer S. L., White P. F. Pharmacokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology. 1988 Sep;69(3):348–356. doi: 10.1097/00000542-198809000-00011. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Himpens B. Cell calcium and its regulation in smooth muscle. FASEB J. 1989 Sep;3(11):2266–2276. doi: 10.1096/fasebj.3.11.2506092. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Vigne P., Barhanin J., Schmid-Alliana A., Frelin C., Lazdunski M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- Vilven J., Coronado R. Opening of dihydropyridine calcium channels in skeletal muscle membranes by inositol trisphosphate. Nature. 1988 Dec 8;336(6199):587–589. doi: 10.1038/336587a0. [DOI] [PubMed] [Google Scholar]
- Xuan Y. T., Wang O. L., Whorton A. R. Regulation of endothelin-induced Ca2+ mobilization in smooth muscle cells by protein kinase C. Am J Physiol. 1994 Jun;266(6 Pt 1):C1560–C1567. doi: 10.1152/ajpcell.1994.266.6.C1560. [DOI] [PubMed] [Google Scholar]
- Xuan Y. T., Wang O. L., Whorton A. R. Thapsigargin stimulates Ca2+ entry in vascular smooth muscle cells: nicardipine-sensitive and -insensitive pathways. Am J Physiol. 1992 May;262(5 Pt 1):C1258–C1265. doi: 10.1152/ajpcell.1992.262.5.C1258. [DOI] [PubMed] [Google Scholar]
- Xuan Y. T., Watkins W. D., Whorton A. R. Regulation of endothelin-mediated calcium mobilization in vascular smooth muscle cells by isoproterenol. Am J Physiol. 1991 Mar;260(3 Pt 1):C492–C502. doi: 10.1152/ajpcell.1991.260.3.C492. [DOI] [PubMed] [Google Scholar]
- Xuan Y. T., Whorton A. R., Watkins W. D. Inhibition by nicardipine of endothelin-mediated inositol phosphate formation and Ca2+ mobilization in smooth muscle cell. Biochem Biophys Res Commun. 1989 Apr 28;160(2):758–764. doi: 10.1016/0006-291x(89)92498-4. [DOI] [PubMed] [Google Scholar]
- Yamanoue T., Brum J. M., Estafanous F. G. Vasodilation and mechanism of action of propofol in porcine coronary artery. Anesthesiology. 1994 Aug;81(2):443–451. doi: 10.1097/00000542-199408000-00023. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


