Abstract
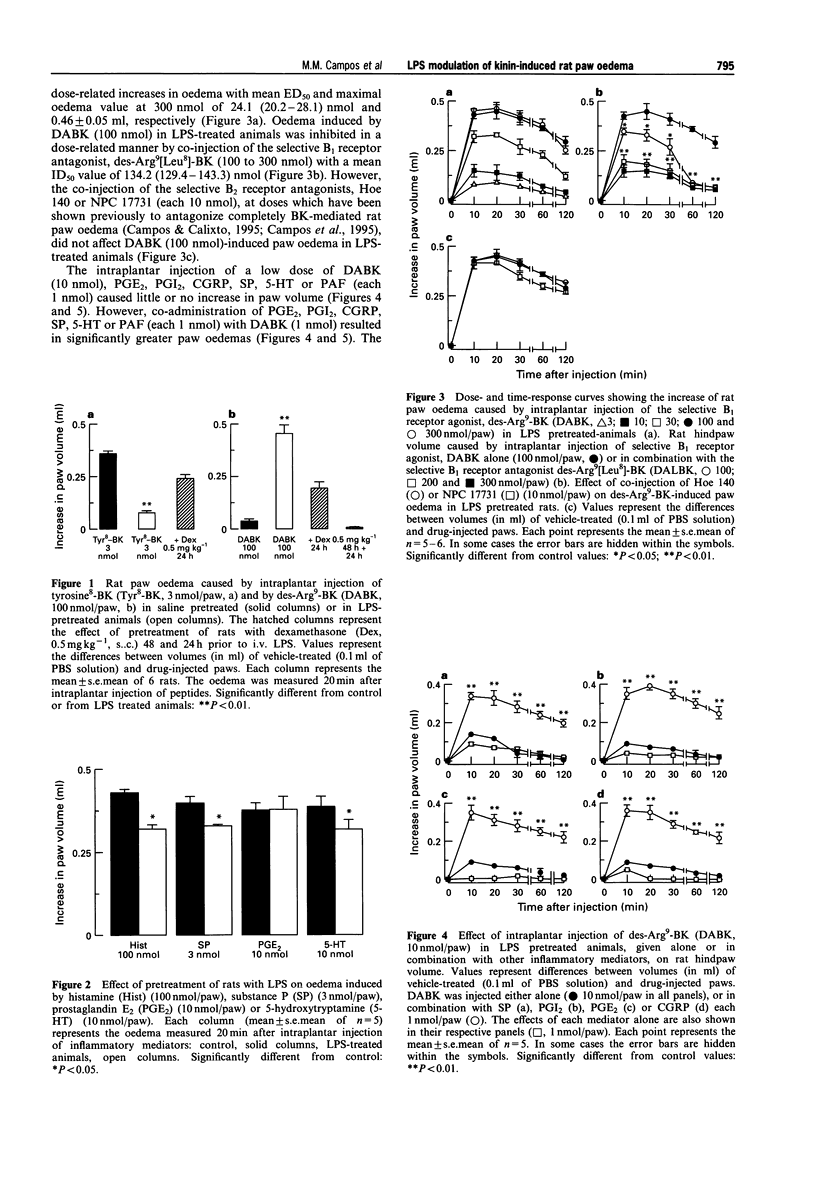
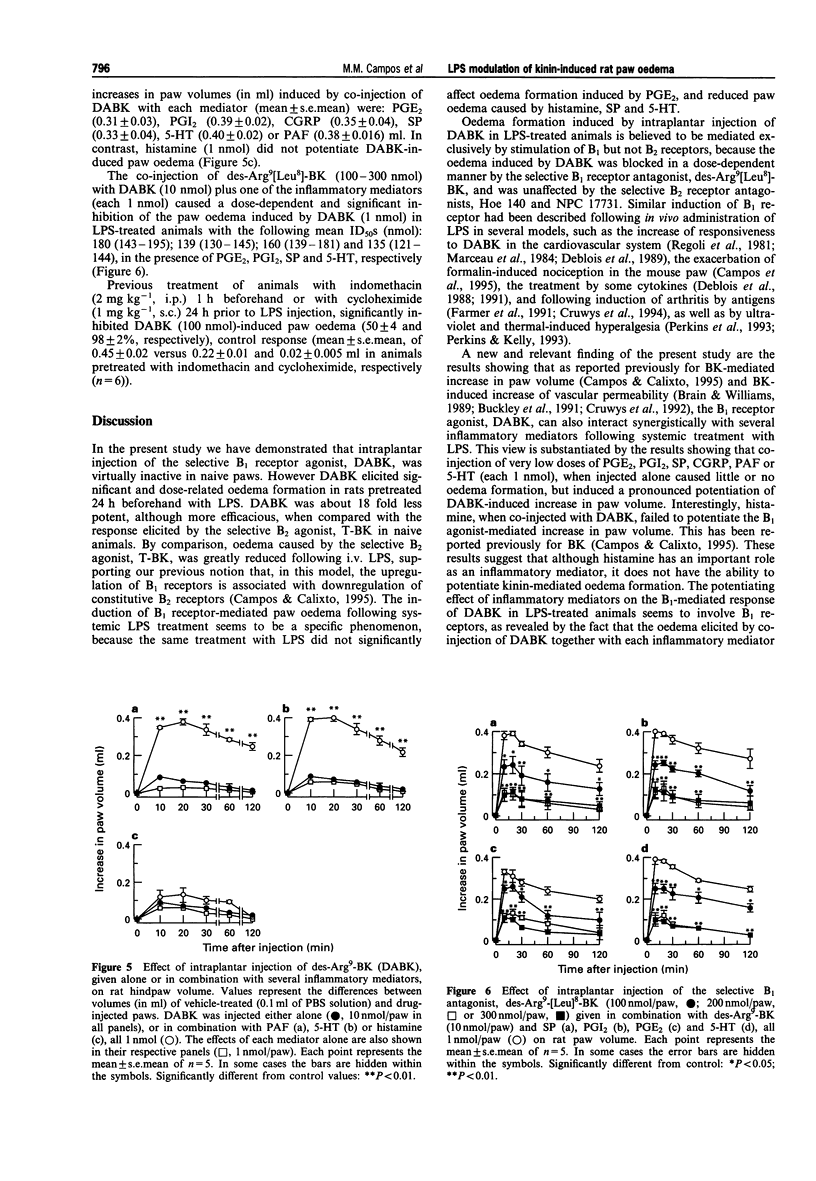
1. The effect of pretreatment with bacterial endotoxin (LPS, 10 micrograms, i.v., 24 h) on the bradykinin B1 and B2 receptor-induced oedema in the rat paw, and the interaction of B1-mediated responses with other inflammatory mediators, was investigated. 2. Intraplantar (i.pl.) injection of the selective B1 agonist, des-Arg9-BK (DABK, 100 nmol) in naive animals pretreated with the angiotensin converting enzyme inhibitor, captopril caused a small increase in paw volume (0.04 +/- 0.003 ml, mean +/- s.e. mean, n = 6), while the B2-selective agonist, tyrosine8-bradykinin (T-BK, 3 nmol) induced marked oedema (0.36 +/- 0.02 ml). However, i.pl. injection of DABK (3-300 nmol) in rats pretreated with LPS (24 h beforehand) resulted in a marked dose- and time-related increase in paw volume, with mean ED50 of 24.1 nmol. In contrast, oedema caused by T-BK (3 nmol) was reduced by 79 +/- 4% in animals treated with LPS when compared with naive animals. 3. Oedema caused by prostaglandin E2 (PGE2, 10 nmol) was unaffected by LPS treatment, while oedema induced by histamine (100 nmol), 5-hydroxytryptamine (5-HT, 10 nmol) and substance P (SP, 3 nmol) was reduced (P < 0.05). 4. The selective B1 antagonist, des-Arg9[Leu8]-BK (100-300 nmol), produced dose-dependent inhibition of DABK (100 nmol)-induced paw oedema in LPS-treated animals with mean IC50 of 134 nmol, while the selective B2 antagonists, Hoe 140 and NPC 17731 (each 10 nmol), had no effect. 5. Treatment of animals with dexamethasone (0.5 mg kg-1, s.c.) 24 or 48 h prior to LPS injection resulted in a graded inhibition of DABK (100 nmol)-induced oedema formation (58 +/- 3 and 82 +/- 2%, respectively), and almost reversed to control value oedema formation induced by T-BK (3 nmol) in LPS-pretreated rats. Cycloheximide (1 mg kg-1, s.c.) or indomethacin (2 mg kg-1, i.p.) pretreatment 24 and 1 h prior to LPS injection, respectively, markedly inhibited DABK (100 nmol)-induced paw oedema (98 +/- 2 and 50 +/- 4%, respectively). 6. Intraplantar injection of submaximal dose of DABK (10 nmol) in LPS-treated rats produced modest paw oedema (0.09 +/- 0.03 ml). However, i.pl. injections of PGE2, prostacyclin (PGI2), calcitonin-gene-related peptide (CGRP), SP, 5-HT, or platelet activating factor (PAF) (each 1 nmol), which alone caused little or no paw oedema, resulted in a potentiation of the DABK-induced oedema. The increases in paw volume (in ml) were: PGE2 + DABK (0.31 +/- 0.03), PGI2 + DABK (0.39 +/- 0.02), CGRP+DABK (0.35 +/- 0.04), DABK+SP (0.33 +/- 0.04), DABK + 5-HT (0.40 +/- 0.02) and DABK+PAF (0.38 +/- 0.016) ml. In contrast, histamine (1 nmol) was ineffective in potentiating the response to DABK. 7. The selective B1 receptor antagonist, DALBK (100-300 nmol), produced dose-dependent inhibition of paw oedema potentiation induced by co-injection of DABK and other mediators with mean ID50S (nmol) of: 180, 160, 139 and 135 in the presence of PGE2, PGI2, SP and 5-HT, respectively. 8. These results demonstrate that DABK-induced increase in paw volume in LPS-treated rats is probably mediated by induction of B1 receptors, associated with downregulation of B2 receptors. The induction of B1 receptors by LPS is sensitive to dexamethasone and cycloheximide treatment and requires activation of cyclo-oxygenase pathway. In addition, B1 receptors, when upregulated following LPS treatment, can interact in a synergistic manner with several inflammatory mediators such as PGI2, PGE2, CGRP, PAF and 5-HT. Such results indicate that induction of the B1 receptor might have a significant pathophysiological role in modulating chronic inflammatory diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bathon J. M., Manning D. C., Goldman D. W., Towns M. C., Proud D. Characterization of kinin receptors on human synovial cells and upregulation of receptor number by interleukin-1. J Pharmacol Exp Ther. 1992 Jan;260(1):384–392. [PubMed] [Google Scholar]
- Bathon J. M., Proud D. Bradykinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:129–162. doi: 10.1146/annurev.pa.31.040191.001021. [DOI] [PubMed] [Google Scholar]
- Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992 Mar;44(1):1–80. [PubMed] [Google Scholar]
- Bouthillier J., Deblois D., Marceau F. Studies on the induction of pharmacological responses to des-Arg9-bradykinin in vitro and in vivo. Br J Pharmacol. 1987 Oct;92(2):257–264. doi: 10.1111/j.1476-5381.1987.tb11319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J. Interactions between the tachykinins and calcitonin gene-related peptide lead to the modulation of oedema formation and blood flow in rat skin. Br J Pharmacol. 1989 May;97(1):77–82. doi: 10.1111/j.1476-5381.1989.tb11926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T. L., Brain S. D., Rampart M., Williams T. J. Time-dependent synergistic interactions between the vasodilator neuropeptide, calcitonin gene-related peptide (CGRP) and mediators of inflammation. Br J Pharmacol. 1991 Jun;103(2):1515–1519. doi: 10.1111/j.1476-5381.1991.tb09819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Connor J. R., Tiffany C. W. The kallikrein-kininogen-kinin system in chronic inflammation. Agents Actions. 1989 Jun;27(3-4):258–260. doi: 10.1007/BF01972790. [DOI] [PubMed] [Google Scholar]
- Cahill M., Fishman J. B., Polgar P. Effect of des arginine9-bradykinin and other bradykinin fragments on the synthesis of prostacyclin and the binding of bradykinin by vascular cells in culture. Agents Actions. 1988 Jul;24(3-4):224–231. doi: 10.1007/BF02028275. [DOI] [PubMed] [Google Scholar]
- Campos M. M., Calixto J. B. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br J Pharmacol. 1995 Mar;114(5):1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockfield S. M., Ramassar V., Noujaim J., van der Meide P. H., Halloran P. F. Regulation of IFN-gamma expression in vivo. IFN-gamma up-regulates expression of its mRNA in normal and lipopolysaccharide-stimulated mice. J Immunol. 1993 Feb 1;150(3):717–725. [PubMed] [Google Scholar]
- Corrêa C. R., Calixto J. B. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol. 1993 Sep;110(1):193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruwys S. C., Garrett N. E., Perkins M. N., Blake D. R., Kidd B. L. The role of bradykinin B1 receptors in the maintenance of intra-articular plasma extravasation in chronic antigen-induced arthritis. Br J Pharmacol. 1994 Nov;113(3):940–944. doi: 10.1111/j.1476-5381.1994.tb17083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruwys S. C., Kidd B. L., Mapp P. I., Walsh D. A., Blake D. R. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharmacol. 1992 Sep;107(1):116–119. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J., Bourdon V., Remacle-Volon G., Adam A. Kinins and peritoneal exudates induced by carrageenin and zymosan in rats. Br J Pharmacol. 1990 Oct;101(2):418–422. doi: 10.1111/j.1476-5381.1990.tb12724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois D., Bouthillier J., Marceau F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit isolated aorta to des-Arg9-bradykinin. Br J Pharmacol. 1988 Apr;93(4):969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Patel I. A., Perkins M. N., Rueff A. Bradykinin-induced activation of nociceptors: receptor and mechanistic studies on the neonatal rat spinal cord-tail preparation in vitro. Br J Pharmacol. 1992 Dec;107(4):1129–1134. doi: 10.1111/j.1476-5381.1992.tb13418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993 Mar;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., McMillan B. A., Meeker S. N., Burch R. M. Induction of vascular smooth muscle bradykinin B1 receptors in vivo during antigen arthritis. Agents Actions. 1991 Sep;34(1-2):191–193. doi: 10.1007/BF01993275. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Bodinier M. C., Chapelain B., Ly S. M., Coussy L., Giraud S., Neliat G., Jean T. Up-regulation of [3H]-des-Arg10-kallidin binding to the bradykinin B1 receptor by interleukin-1 beta in isolated smooth muscle cells: correlation with B1 agonist-induced PGI2 production. Br J Pharmacol. 1994 Oct;113(2):389–394. doi: 10.1111/j.1476-5381.1994.tb17001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Arnold J., Rordorf C., Henn R., Vosbeck K. Interferon-gamma overcomes the glucocorticoid-mediated and the interleukin-4-mediated inhibition of interleukin-1 beta synthesis in human monocytes. Lymphokine Cytokine Res. 1993 Oct;12(5):271–278. [PubMed] [Google Scholar]
- Hargreaves K. M., Troullos E. S., Dionne R. A., Schmidt E. A., Schafer S. C., Joris J. L. Bradykinin is increased during acute and chronic inflammation: therapeutic implications. Clin Pharmacol Ther. 1988 Dec;44(6):613–621. doi: 10.1038/clpt.1988.202. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Borkowski J. A., Macneil T., Stonesifer G. Y., Fraher J., Strader C. D., Ransom R. W. Differential pharmacology of cloned human and mouse B2 bradykinin receptors. Mol Pharmacol. 1994 Jan;45(1):1–8. [PubMed] [Google Scholar]
- Hess J. F., Borkowski J. A., Young G. S., Strader C. D., Ransom R. W. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- Huleihel M., Douvdevani A., Segal S., Apte R. N. Different regulatory levels are involved in the generation of hemopoietic cytokines (CSFs and IL-6) in fibroblasts stimulated by inflammatory products. Cytokine. 1993 Jan;5(1):47–56. doi: 10.1016/1043-4666(93)90023-x. [DOI] [PubMed] [Google Scholar]
- Lerner U. H., Brunius G., Modeer T. On the signal transducing mechanisms involved in the synergistic interaction between interleukin-1 and bradykinin on prostaglandin biosynthesis in human gingival fibroblasts. Biosci Rep. 1992 Aug;12(4):263–271. doi: 10.1007/BF01122798. [DOI] [PubMed] [Google Scholar]
- Lerner U. H., Modéer T. Bradykinin B1 and B2 receptor agonists synergistically potentiate interleukin-1-induced prostaglandin biosynthesis in human gingival fibroblasts. Inflammation. 1991 Dec;15(6):427–436. doi: 10.1007/BF00923340. [DOI] [PubMed] [Google Scholar]
- Marceau F., Barabé J., St-Pierre S., Regoli D. Kinin receptors in experimental inflammation. Can J Physiol Pharmacol. 1980 May;58(5):536–542. doi: 10.1139/y80-088. [DOI] [PubMed] [Google Scholar]
- Marceau F., Lussier A., Regoli D., Giroud J. P. Pharmacology of kinins: their relevance to tissue injury and inflammation. Gen Pharmacol. 1983;14(2):209–229. doi: 10.1016/0306-3623(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Marceau F., Lussier A., St-Pierre S. Selective induction of cardiovascular responses to des-Arg9-bradykinin by bacterial endotoxin. Pharmacology. 1984;29(2):70–74. doi: 10.1159/000137994. [DOI] [PubMed] [Google Scholar]
- McEachern A. E., Shelton E. R., Bhakta S., Obernolte R., Bach C., Zuppan P., Fujisaki J., Aldrich R. W., Jarnagin K. Expression cloning of a rat B2 bradykinin receptor. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke J. G., Borkowski J. A., Bierilo K. K., MacNeil T., Derrick A. W., Schneck K. A., Ransom R. W., Strader C. D., Linemeyer D. L., Hess J. F. Expression cloning of a human B1 bradykinin receptor. J Biol Chem. 1994 Aug 26;269(34):21583–21586. [PubMed] [Google Scholar]
- Ochalski S. J., Hartman D. A., Belfast M. T., Walter T. L., Glaser K. B., Carlson R. P. Inhibition of endotoxin-induced hypothermia and serum TNF-alpha levels in CD-1 mice by various pharmacological agents. Agents Actions. 1993;39(Spec No):C52–C54. doi: 10.1007/BF01972718. [DOI] [PubMed] [Google Scholar]
- Ogle C. K., Guo X., Szczur K., Hartmann S., Ogle J. D. Production of tumor necrosis factor, interleukin-6 and prostaglandin E2 by LPS-stimulated rat bone marrow macrophages after thermal injury: effect of indomethacin. Inflammation. 1994 Apr;18(2):175–185. doi: 10.1007/BF01534558. [DOI] [PubMed] [Google Scholar]
- Pang G., Couch L., Batey R., Clancy R., Cripps A. GM-CSF, IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1 alpha and TNF-alpha. Clin Exp Immunol. 1994 Jun;96(3):437–443. doi: 10.1111/j.1365-2249.1994.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M. N., Campbell E., Dray A. Antinociceptive activity of the bradykinin B1 and B2 receptor antagonists, des-Arg9, [Leu8]-BK and HOE 140, in two models of persistent hyperalgesia in the rat. Pain. 1993 May;53(2):191–197. doi: 10.1016/0304-3959(93)90080-9. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Kelly D. Induction of bradykinin B1 receptors in vivo in a model of ultra-violet irradiation-induced thermal hyperalgesia in the rat. Br J Pharmacol. 1993 Dec;110(4):1441–1444. doi: 10.1111/j.1476-5381.1993.tb13982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. J., Slynn G., Thomas C., Hopkins B., Briggs I., Graham A. Human bradykinin B2 receptor: nucleotide sequence analysis and assignment to chromosome 14. Genomics. 1993 Feb;15(2):435–438. doi: 10.1006/geno.1993.1084. [DOI] [PubMed] [Google Scholar]
- Proud D., Kaplan A. P. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- Regoli D. C., Marceau F., Lavigne J. Induction of beta 1-receptors for kinins in the rabbit by a bacterial lipopolysaccharide. Eur J Pharmacol. 1981 Apr 24;71(1):105–115. doi: 10.1016/0014-2999(81)90391-5. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Regoli D., Marceau F., Barabé J. De novo formation of vascular receptors for bradykinin. Can J Physiol Pharmacol. 1978 Aug;56(4):674–677. doi: 10.1139/y78-109. [DOI] [PubMed] [Google Scholar]
- Tiffany C. W., Burch R. M. Bradykinin stimulates tumor necrosis factor and interleukin-1 release from macrophages. FEBS Lett. 1989 Apr 24;247(2):189–192. doi: 10.1016/0014-5793(89)81331-6. [DOI] [PubMed] [Google Scholar]
- Toda N., Bian K., Akiba T., Okamura T. Heterogeneity in mechanisms of bradykinin action in canine isolated blood vessels. Eur J Pharmacol. 1987 Mar 31;135(3):321–329. doi: 10.1016/0014-2999(87)90681-9. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Guo K., Yin S., del Castillo J., Yi E. S., Thompson R. C., Eisenberg S. P. Endotoxin-induced cytokine gene expression in vivo. IV. Expression of interleukin-1 alpha/beta and interleukin-1 receptor antagonist mRNA during endotoxemia and during endotoxin-initiated local acute inflammation. Am J Pathol. 1992 Jul;141(1):61–68. [PMC free article] [PubMed] [Google Scholar]
- Whalley E. T., Fritz H., Geiger R. Kinin receptors and angiotensin converting enzyme in rabbits basilar arteries. Naunyn Schmiedebergs Arch Pharmacol. 1983 Dec;324(4):296–301. doi: 10.1007/BF00502627. [DOI] [PubMed] [Google Scholar]
- deBlois D., Bouthillier J., Marceau F. Pharmacological modulation of the up-regulated responses to des-Arg9-bradykinin in vivo and in vitro. Immunopharmacology. 1989 May-Jun;17(3):187–198. doi: 10.1016/0162-3109(89)90047-7. [DOI] [PubMed] [Google Scholar]


