Abstract
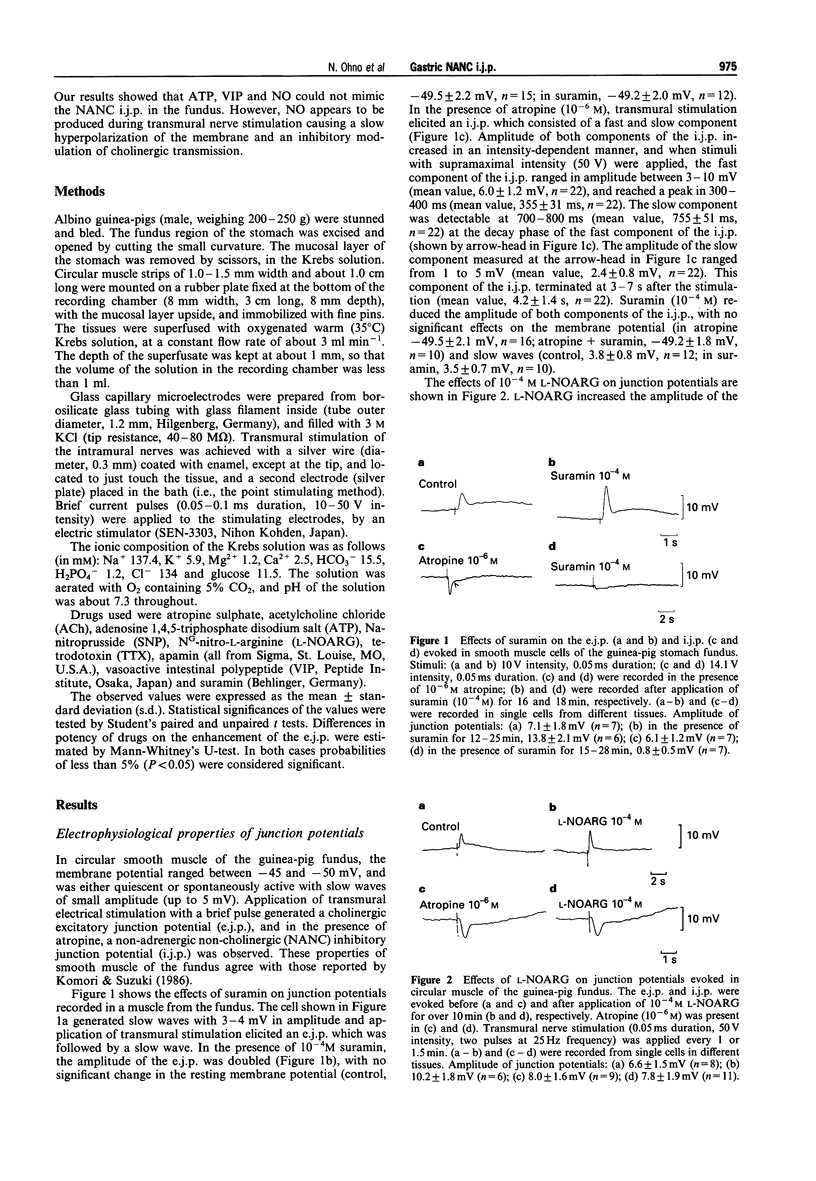
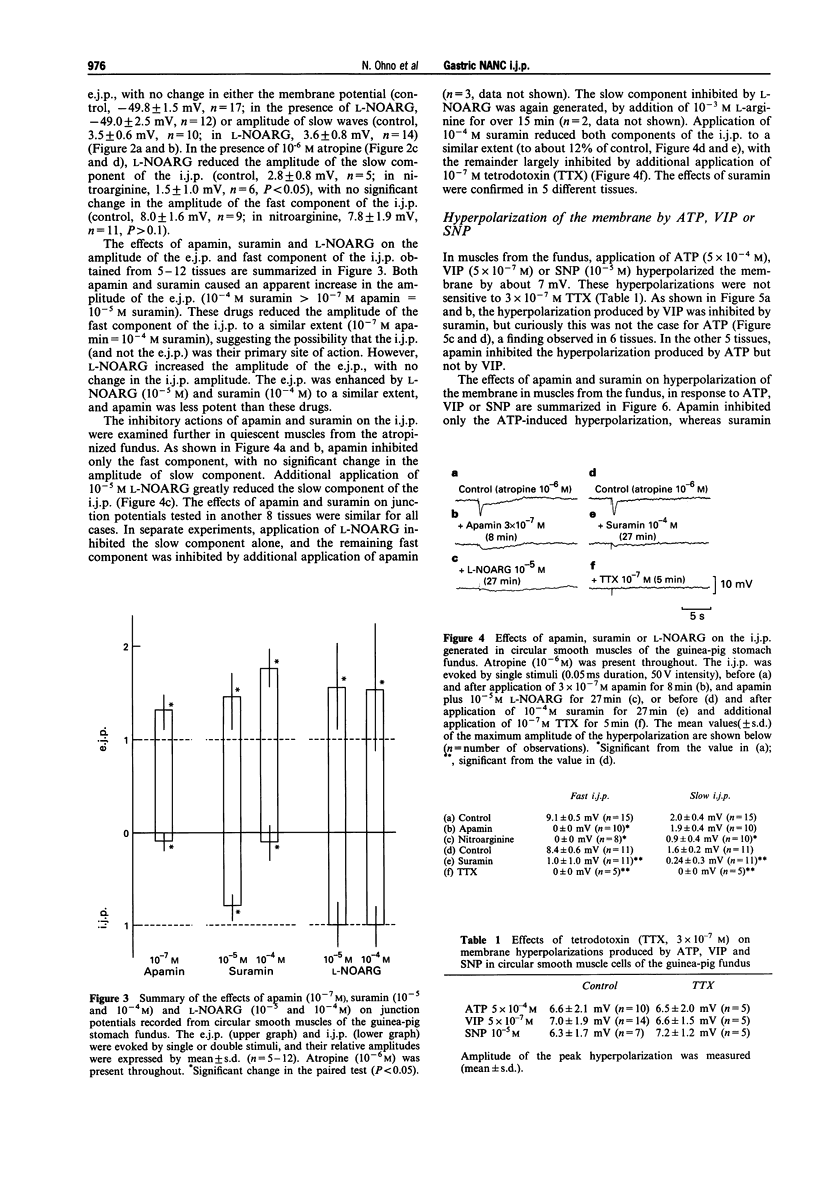
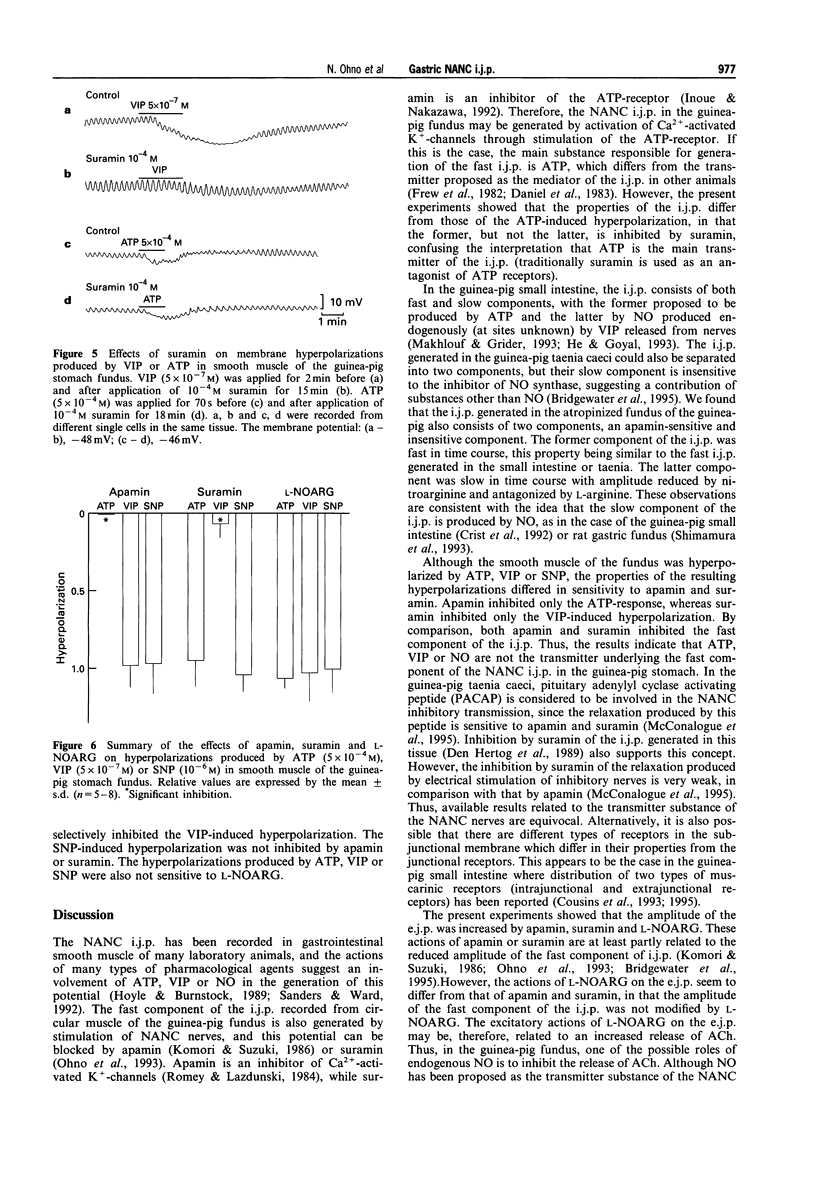
1. In circular smooth muscle of the guinea-pig gastric fundus, transmural nerve stimulation evoked a cholinergic excitatory junction potential (e.j.p.), and blockade of the e.j.p. by atropine revealed a non-adrenergic non-cholinergic (NANC) inhibitory junction potential (i.j.p.). 2. The amplitude of the e.j.p. was increased by apamin, suramin or NGnitro-L-arginine (L-NOARG), with no significant change in the membrane potential. 3. The i.j.p. consisted of two components (fast and slow); apamin inhibited the former, nitroarginine inhibited the latter, and suramin inhibited both components. 4. Apamin inhibited the hyperpolarization produced by adenosine 5'-triphosphate (ATP) but not by vasoactive intestinal polypeptide (VIP). Suramin inhibited the hyperpolarization produced by VIP but not by ATP. The sodium nitroprusside (SNP)-induced hyperpolarization was not blocked by apamin or suramin. L-NOARG or tetrodotoxin did not inhibit the hyperpolarization produced by ATP, VIP or SNP. 5. The data did not support the hypothesis that ATP, VIP or nitric oxide (NO) is the main transmitter responsible for generation of the NANC i.j.p. in the fundus. 6. Actions of L-NOARG suggest that endogenous NO may be involved in junctional transmission, mainly as an inhibitory modulator of cholinergic transmission.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridgewater M., Cunnane T. C., Brading A. F. Characteristic features of inhibitory junction potentials evoked by single stimuli in the guinea-pig isolated taenia caeci. J Physiol. 1995 May 15;485(Pt 1):145–155. doi: 10.1113/jphysiol.1995.sp020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. J Physiol. 1986 May;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christinck F., Jury J., Cayabyab F., Daniel E. E. Nitric oxide may be the final mediator of nonadrenergic, noncholinergic inhibitory junction potentials in the gut. Can J Physiol Pharmacol. 1991 Oct;69(10):1448–1458. doi: 10.1139/y91-217. [DOI] [PubMed] [Google Scholar]
- Cousins H. M., Edwards F. R., Hirst G. D. Neuronally released and applied acetylcholine on the longitudinal muscle of the guinea-pig ileum. Neuroscience. 1995 Mar;65(1):193–207. doi: 10.1016/0306-4522(94)00466-i. [DOI] [PubMed] [Google Scholar]
- Cousins H. M., Edwards F. R., Hirst G. D., Wendt I. R. Cholinergic neuromuscular transmission in the longitudinal muscle of the guinea-pig ileum. J Physiol. 1993 Nov;471:61–86. doi: 10.1113/jphysiol.1993.sp019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist J. R., He X. D., Goyal R. K. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992 Feb;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel H. H., Thornbury K. D., Ward S. M., Sanders K. M. Involvement of nitric oxide synthetic pathway in inhibitory junction potentials in canine proximal colon. Am J Physiol. 1991 May;260(5 Pt 1):G789–G792. doi: 10.1152/ajpgi.1991.260.5.G789. [DOI] [PubMed] [Google Scholar]
- Den Hertog A., Van den Akker J., Nelemans A. Suramin and the inhibitory junction potential in taenia caeci of the guinea-pig. Eur J Pharmacol. 1989 Dec 7;173(2-3):207–209. doi: 10.1016/0014-2999(89)90522-0. [DOI] [PubMed] [Google Scholar]
- Frew R., Lundy P. M. Evidence against ATP being the nonadrenergic, noncholinergic inhibitory transmitter in guinea pig stomach. Eur J Pharmacol. 1982 Jul 9;81(2):333–336. doi: 10.1016/0014-2999(82)90453-8. [DOI] [PubMed] [Google Scholar]
- Grider J. R., Makhlouf G. M. Vasoactive intestinal peptide. Transmitter of inhibitory motor neurons of the gut. Ann N Y Acad Sci. 1988;527:369–377. doi: 10.1111/j.1749-6632.1988.tb26993.x. [DOI] [PubMed] [Google Scholar]
- Grider J. R., Murthy K. S., Jin J. G., Makhlouf G. M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol. 1992 Apr;262(4 Pt 1):G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- He X. D., Goyal R. K. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. J Physiol. 1993 Feb;461:485–499. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Kurokawa A., Ohga A., Ohta T., Sawabe K. Mechanical, electrical and cyclic nucleotide responses to peptide VIP and inhibitory nerve stimulation in rat stomach. J Physiol. 1990 Nov;430:337–353. doi: 10.1113/jphysiol.1990.sp018294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Lian Q., Carl A., Kuriyama H. S-nitrosocysteine, but not sodium nitroprusside, produces apamin-sensitive hyperpolarization in rat gastric fundus. Br J Pharmacol. 1993 Jun;109(2):415–423. doi: 10.1111/j.1476-5381.1993.tb13585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Distribution and properties of excitatory and inhibitory junction potentials in circular muscle of the guinea-pig stomach. J Physiol. 1986 Jan;370:339–355. doi: 10.1113/jphysiol.1986.sp015938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConalogue K., Furness J. B., Vremec M. A., Holst J. J., Tornøe K., Marley P. D. Histochemical, pharmacological, biochemical and chromatographic evidence that pituitary adenylyl cyclase activating peptide is involved in inhibitory neurotransmission in the taenia of the guinea-pig caecum. J Auton Nerv Syst. 1995 Jan 3;50(3):311–322. doi: 10.1016/0165-1838(94)00102-p. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Niel J. P., Bywater R. A., Taylor G. S. Apamin-resistant post-stimulus hyperpolarization in the circular muscle of the guinea-pig ileum. J Auton Nerv Syst. 1983 Nov;9(2-3):565–569. doi: 10.1016/0165-1838(83)90014-0. [DOI] [PubMed] [Google Scholar]
- Ohno N., Ito K. M., Yamamoto Y., Suzuki H. Suramin selectively inhibits the non-adrenergic non-cholinergic inhibitory junction potential in the guinea-pig stomach. Eur J Pharmacol. 1993 Nov 2;249(1):121–123. doi: 10.1016/0014-2999(93)90671-4. [DOI] [PubMed] [Google Scholar]
- Romey G., Lazdunski M. The coexistence in rat muscle cells of two distinct classes of Ca2+-dependent K+ channels with different pharmacological properties and different physiological functions. Biochem Biophys Res Commun. 1984 Jan 30;118(2):669–674. doi: 10.1016/0006-291x(84)91355-x. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992 Mar;262(3 Pt 1):G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Shimamura K., Fujisawa A., Toda N., Sunano S. Effects of NG-nitro-L-arginine on electrical and mechanical responses to stimulation of non-adrenergic, non-cholinergic inhibitory nerves in circular muscle of the rat gastric fundus. Eur J Pharmacol. 1993 Jan 26;231(1):103–109. doi: 10.1016/0014-2999(93)90690-j. [DOI] [PubMed] [Google Scholar]


