Abstract
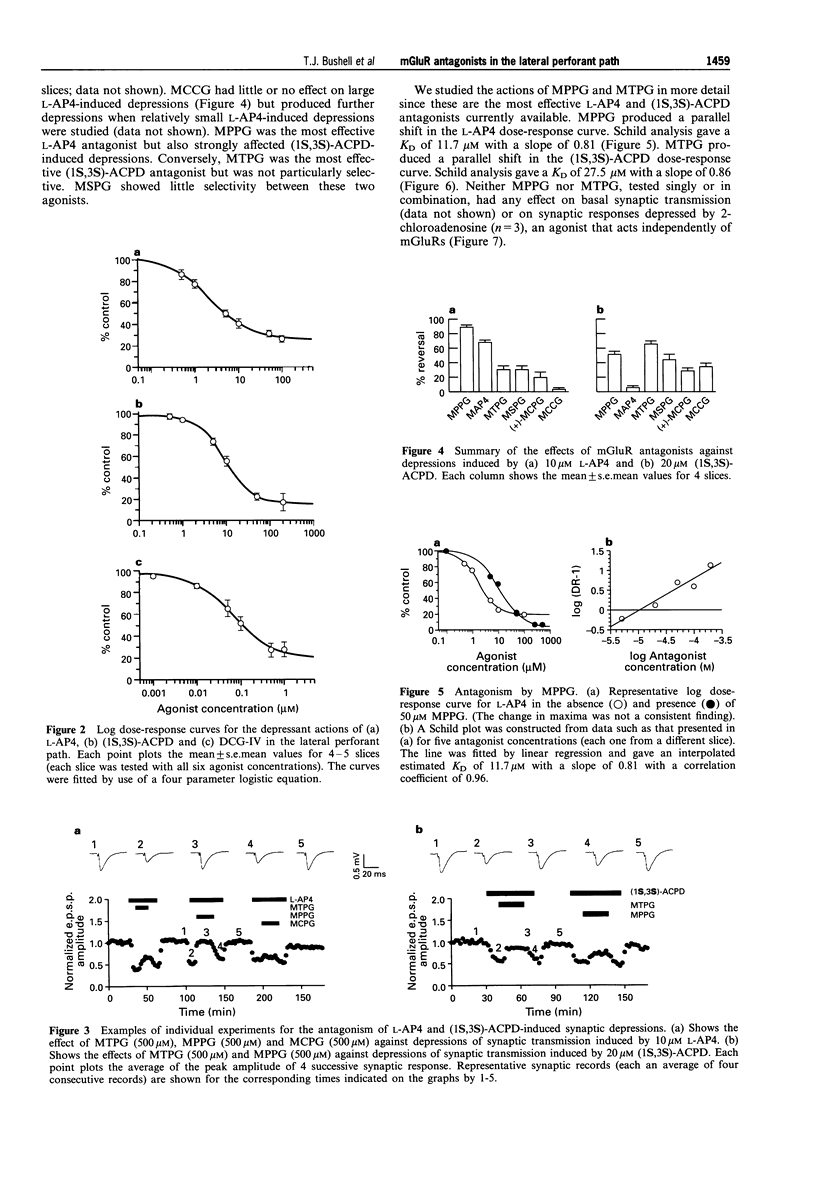
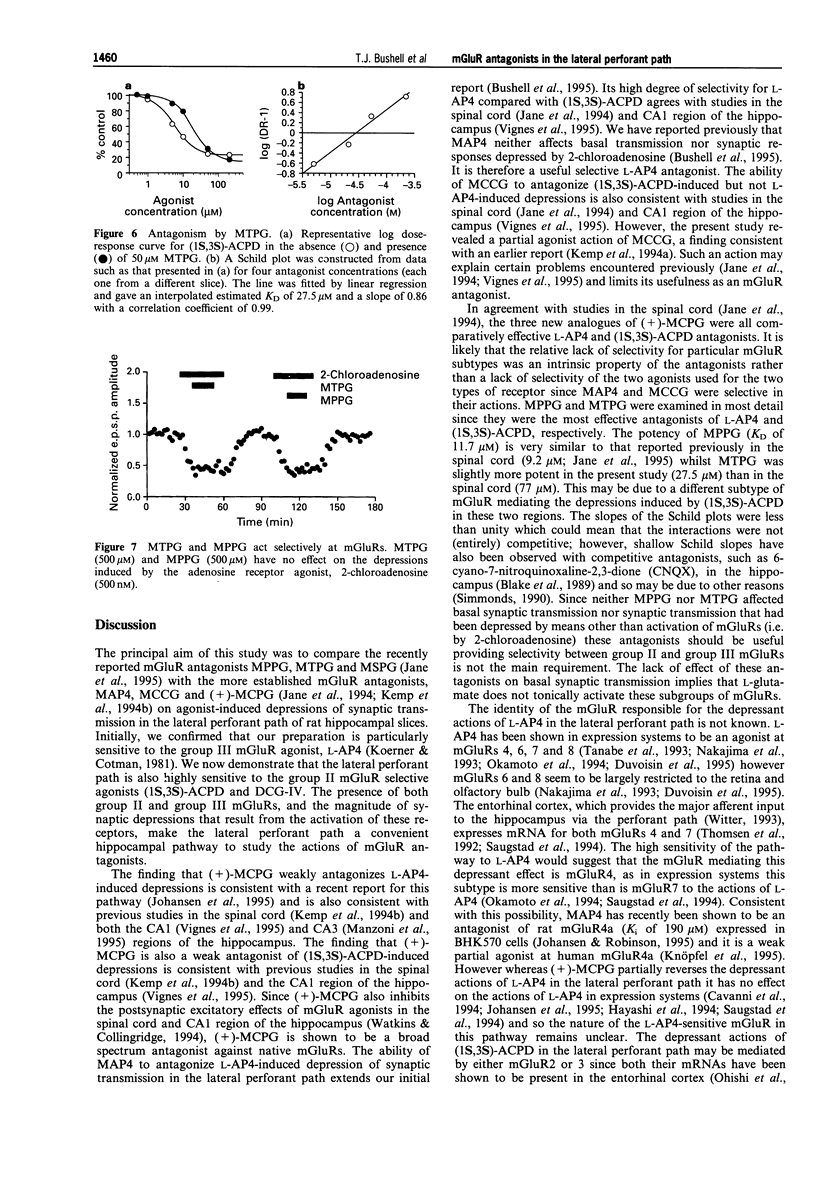
1. An understanding of the physiological and pathological roles of metabotropic glutamate receptors (mGluRs) is currently hampered by the lack of selective antagonists. Standard extracellular recording techniques were used to investigate the activity of recently reported mGluR antagonists on agonist-induced depressions of synaptic transmission in the lateral perforant path of hippocampal slices obtained from 12-16 day-old rats. 2. The group III specific mGluR agonist, (S)-2-amino-4-phosphonobutanoate (L-AP4) depressed basal synaptic transmission in a reversible and dose-dependent manner. The mean (+/-s.e. mean) depression obtained with 100 microM L-AP4 (the maximum concentration tested) was 74 +/- 3% and the IC50 value was 3 +/- 1 microM (n = 5). 3. The selective group II mGluR agonists, (1S,3S)-1-aminocyclopentane-1, 3-dicarboxylate ((1S,3s)-ACPD) and (2S, 1'R, 2'R, 3'R)-2-(2',3'-dicarboxycyclopropyl)glycine (DCG-IV) also depressed basal synaptic transmission in a reversible and dose-dependent manner. The mean depression obtained with 200 microM (1S,3S)-ACPD was 83 +/- 8% and the IC50 value was 12 +/- 3 microM (n = 5). The mean depression obtained with 1 microM DCG-IV was 73 +/- 7% and the IC50 value was 88 +/- 15 nM (n = 4). 4. Synaptic depressions induced by the actions of 20 microM (1S,3S)-ACPD and 10 microM L-AP4 were antagonized by the mGluR antagonists (+)-alpha-methyl-4-carboxyphenylglycine ((+)-MCPG), (S)-2-methyl-2-amino-4-phosphonobutanoate (MAP4), (2S,1'S,2'S)-2-methyl-2(2'-carboxycyclopropyl)glycine (MCCG), (RS)-alpha-methyl-4-tetrazolylphenylglycine (MTPG), (RS)-alpha-methyl-4-sulphonophenylglycine (MSPG) and (RS)-alpha-methyl-4-phosphonophenylglycine (MPPG) (all tested at 500 microM). 5. (+)-MCPG was a weak antagonist of both L-AP4 and (1S,3S)-ACPD-induced depressions. MCCG was selective towards (1S,3S)-ACPD, but analysis of its effects were complicated by apparent partial agonist activity. MAP4 showed good selectivity for L-AP4-induced effects. 6. The most effective antagonist tested against 10 microM L-AP4 was MPPG (mean reversal 90 +/- 3%; n = 4). In contrast, the most effective antagonist tested against 20 microM (1S,3S)-ACPD induced depressions was MTPG (mean reversal 64 +/- 4%; n = 4). Both antagonists produced parallel shifts in agonist dose-response curves. Schild analysis yielded estimated KD values of 11.7 microM and 27.5 microM, respectively. Neither antagonist had any effect on basal transmission or on depressions induced by the adenosine receptor agonist, 2-chloroadenosine (500 nM; n = 3). 7. We conclude that both group II and group III mGluRs can mediate synaptic depressions induced by mGluR agonists in the lateral perforant path. The mGlur antagonists MTPG, MPPG and MAP4 should be useful in determining the roles of group II and III mGluRs in the central nervous system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashir Zafar I., Collingridge Graham L. NMDA Receptor-dependent Transient Homo- and Heterosynaptic Depression in Picrotoxin-treated Hippocampal Slices. Eur J Neurosci. 1992;4(6):485–490. doi: 10.1111/j.1460-9568.1992.tb00898.x. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Yates R. G., Brown M. W., Collingridge G. L. 6-Cyano-7-nitroquinoxaline-2,3-dione as an excitatory amino acid antagonist in area CA1 of rat hippocampus. Br J Pharmacol. 1989 May;97(1):71–76. doi: 10.1111/j.1476-5381.1989.tb11925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell T. J., Jane D. E., Tse H. W., Watkins J. C., Davies C. H., Garthwaite J., Collingridge G. L. Antagonism of the synaptic depressant actions of L-AP4 in the lateral perforant path by MAP4. Neuropharmacology. 1995 Feb;34(2):239–241. doi: 10.1016/0028-3908(95)00012-u. [DOI] [PubMed] [Google Scholar]
- Cavanni P., Pinnola V., Mugnaini M., Trist D., Van Amsterdam F. T., Ferraguti F. Pharmacological analysis of carboxyphenylglycines at metabotropic glutamate receptors. Eur J Pharmacol. 1994 Sep 15;269(1):9–15. doi: 10.1016/0922-4106(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. The action of some analogues of the excitatory amino acids in the dentate gyrus of the rat. Can J Physiol Pharmacol. 1984 Apr;62(4):424–429. doi: 10.1139/y84-067. [DOI] [PubMed] [Google Scholar]
- Duvoisin R. M., Zhang C., Ramonell K. A novel metabotropic glutamate receptor expressed in the retina and olfactory bulb. J Neurosci. 1995 Apr;15(4):3075–3083. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M., Standaert D. G., Testa C. M., Penney J. B., Jr, Young A. B. Differential expression of metabotropic glutamate receptors in the hippocampus and entorhinal cortex of the rat. Brain Res Mol Brain Res. 1994 Feb;21(3-4):283–292. doi: 10.1016/0169-328x(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Sekiyama N., Nakanishi S., Jane D. E., Sunter D. C., Birse E. F., Udvarhelyi P. M., Watkins J. C. Analysis of agonist and antagonist activities of phenylglycine derivatives for different cloned metabotropic glutamate receptor subtypes. J Neurosci. 1994 May;14(5 Pt 2):3370–3377. doi: 10.1523/JNEUROSCI.14-05-03370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida M., Saitoh T., Shimamoto K., Ohfune Y., Shinozaki H. A novel metabotropic glutamate receptor agonist: marked depression of monosynaptic excitation in the newborn rat isolated spinal cord. Br J Pharmacol. 1993 Aug;109(4):1169–1177. doi: 10.1111/j.1476-5381.1993.tb13745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane D. E., Jones P. L., Pook P. C., Tse H. W., Watkins J. C. Actions of two new antagonists showing selectivity for different sub-types of metabotropic glutamate receptor in the neonatal rat spinal cord. Br J Pharmacol. 1994 Jul;112(3):809–816. doi: 10.1111/j.1476-5381.1994.tb13151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane D. E., Pittaway K., Sunter D. C., Thomas N. K., Watkins J. C. New phenylglycine derivatives with potent and selective antagonist activity at presynaptic glutamate receptors in neonatal rat spinal cord. Neuropharmacology. 1995 Aug;34(8):851–856. doi: 10.1016/0028-3908(95)00063-c. [DOI] [PubMed] [Google Scholar]
- Johansen P. A., Chase L. A., Sinor A. D., Koerner J. F., Johnson R. L., Robinson M. B. Type 4a metabotropic glutamate receptor: identification of new potent agonists and differentiation from the L-(+)-2-amino-4-phosphonobutanoic acid-sensitive receptor in the lateral perforant pathway in rats. Mol Pharmacol. 1995 Jul;48(1):140–149. [PubMed] [Google Scholar]
- Kemp M., Roberts P., Pook P., Jane D., Jones A., Jones P., Sunter D., Udvarhelyi P., Watkins J. Antagonism of presynaptically mediated depressant responses and cyclic AMP-coupled metabotropic glutamate receptors. Eur J Pharmacol. 1994 Jan 15;266(2):187–192. doi: 10.1016/0922-4106(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Knöpfel T., Lukic S., Leonard T., Flor P. J., Kuhn R., Gasparini F. Pharmacological characterization of MCCG and MAP4 at the mGluR1b, mGluR2 and mGluR4a human metabotropic glutamate receptor subtypes. Neuropharmacology. 1995 Aug;34(8):1099–1102. doi: 10.1016/0028-3908(95)00111-i. [DOI] [PubMed] [Google Scholar]
- Koerner J. F., Cotman C. W. Micromolar L-2-amino-4-phosphonobutyric acid selectively inhibits perforant path synapses from lateral entorhinal cortex. Brain Res. 1981 Jul 6;216(1):192–198. doi: 10.1016/0006-8993(81)91288-9. [DOI] [PubMed] [Google Scholar]
- Manzoni O. J., Castillo P. E., Nicoll R. A. Pharmacology of metabotropic glutamate receptors at the mossy fiber synapses of the guinea pig hippocampus. Neuropharmacology. 1995 Aug;34(8):965–971. doi: 10.1016/0028-3908(95)00060-j. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res. 1980 Oct 13;199(1):1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Iwakabe H., Akazawa C., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993 Jun 5;268(16):11868–11873. [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Ohishi H., Shigemoto R., Nakanishi S., Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993 Apr;53(4):1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Okamoto N., Hori S., Akazawa C., Hayashi Y., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol Chem. 1994 Jan 14;269(2):1231–1236. [PubMed] [Google Scholar]
- Pin J. P., Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995 Jan;34(1):1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pook P. C., Sunter D. C., Udvarhelyi P. M., Watkins J. C. Evidence for presynaptic depression of monosynaptic excitation in neonatal rat motoneurones by (1S,3S)- and (1S,3R)-ACPD. Exp Physiol. 1992 May;77(3):529–532. doi: 10.1113/expphysiol.1992.sp003617. [DOI] [PubMed] [Google Scholar]
- Saugstad J. A., Kinzie J. M., Mulvihill E. R., Segerson T. P., Westbrook G. L. Cloning and expression of a new member of the L-2-amino-4-phosphonobutyric acid-sensitive class of metabotropic glutamate receptors. Mol Pharmacol. 1994 Mar;45(3):367–372. [PubMed] [Google Scholar]
- Tanabe Y., Nomura A., Masu M., Shigemoto R., Mizuno N., Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci. 1993 Apr;13(4):1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C., Kristensen P., Mulvihill E., Haldeman B., Suzdak P. D. L-2-amino-4-phosphonobutyrate (L-AP4) is an agonist at the type IV metabotropic glutamate receptor which is negatively coupled to adenylate cyclase. Eur J Pharmacol. 1992 Nov 2;227(3):361–362. doi: 10.1016/0922-4106(92)90018-q. [DOI] [PubMed] [Google Scholar]
- Vignes M., Clarke V. R., Davies C. H., Chambers A., Jane D. E., Watkins J. C., Collingridge G. L. Pharmacological evidence for an involvement of group II and group III mGluRs in the presynaptic regulation of excitatory synaptic responses in the CA1 region of rat hippocampal slices. Neuropharmacology. 1995 Aug;34(8):973–982. doi: 10.1016/0028-3908(95)00093-l. [DOI] [PubMed] [Google Scholar]
- Watkins J., Collingridge G. Phenylglycine derivatives as antagonists of metabotropic glutamate receptors. Trends Pharmacol Sci. 1994 Sep;15(9):333–342. doi: 10.1016/0165-6147(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Witter M. P. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3(Spec No):33–44. [PubMed] [Google Scholar]


