Abstract
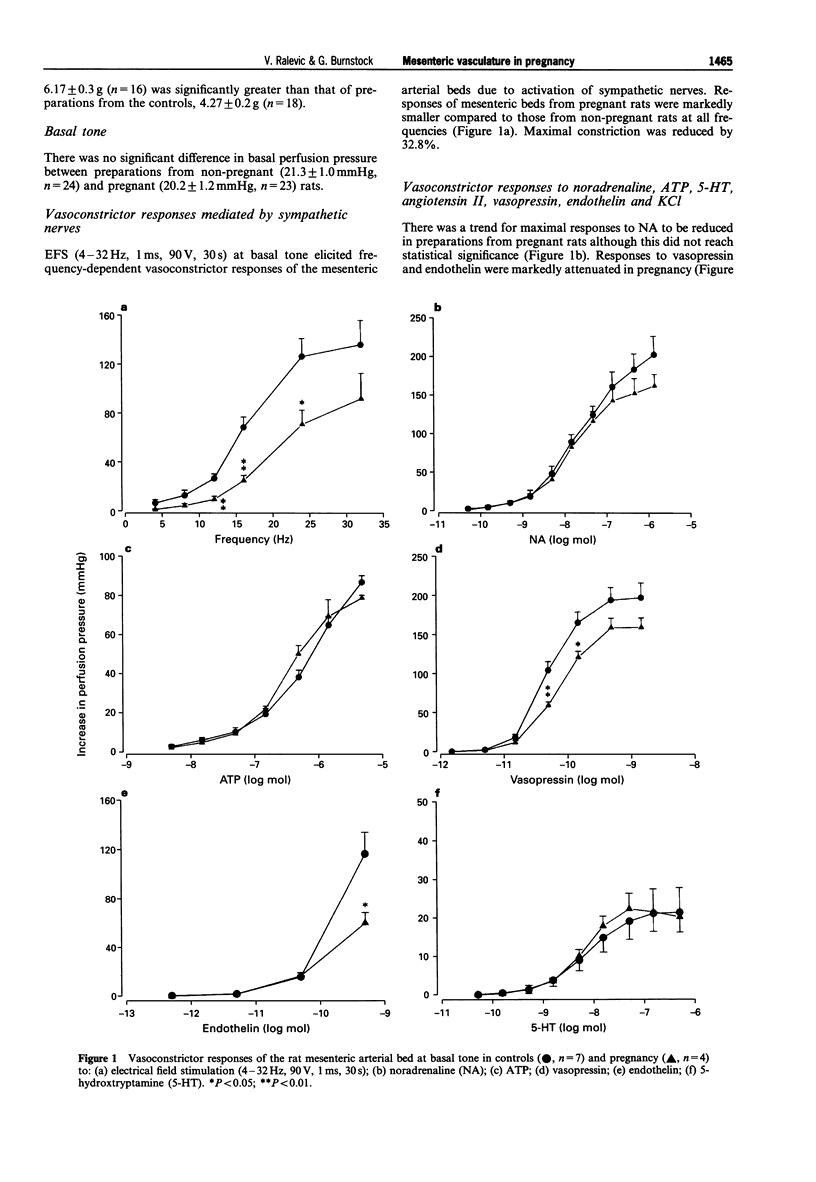
1. The effects of pregnancy on mesenteric arterial function were examined in constantly perfused (5 ml min-1) mesenteric arterial beds isolated from 21-day pregnant rats. The function of sympathetic and sensory-motor perivascular nerves, endothelium and smooth muscle was examined. The role of nitric oxide and prostaglandins in vasoconstrictor function was tested by use of NG-nitro-L-arginine methyl ester (L-NAME; 100 microM) and indomethacin (10 microM), respectively. 2. Electrical field stimulation (EFS; 4-32 Hz, 1 ms, 90V, 30s) at basal tone elicited frequency-dependent vasoconstriction which was markedly reduced in preparations from pregnant rats at all frequencies. Vasoconstrictor responses to vasopressin and endothelin were also reduced in pregnancy and there was a trend towards a reduction in maximal responses to noradrenaline (NA). In contrast, there was no difference in vasoconstrictor responses to ATP, 5-hydroxytryptamine (5-HT) or angiotension II. 3. L-NAME (100 microM) augmented responses to EFS, NA, ATP and vasopressin in control mesenteric arterial preparations. In contrast, L-NAME augmented responses only to EFS in pregnancy, having no significant effect on responses to NA, ATP and vasopressin. 4. Indomethacin (10 microM) attenuated responses to NA and vasopressin, but not to EFS, in controls and in pregnancy. Responses to ATP were attenuated by indomethacin in controls but not in pregnancy. 5. Mesenteric preparations from pregnant rats were resistant to having tone raised by continuous perfusion with methoxamine. Despite an approximately 10 fold greater concentration of methoxamine, there was a significantly smaller increase in tone in preparations from pregnant, 34.27 +/- 4.8 mmHg (n = 11) compared to control, 65.92 +/- 5.4 mmHg (n = 11), rats. EFS (4-12 Hz, 60 V, 0.1 ms, 30s) in the presence of guanethidine (5 microM) to block sympathetic neurotransmission elicited frequency-dependent vasodilatation due to activation of sensory-motor nerves. Percentage relaxations were similar in preparations from pregnant and non-pregnant rats. 6. Dose-dependent endothelium-dependent vasodilatations to acetylcholine and ATP were similar in preparations from pregnant and non-pregnant rats. Endothelium-independent vasodilatation to sodium nitroprusside and to calcitonin gene-related peptide were also similar between the two groups. 7. There was no significant difference in the basal perfusion pressure of mesenteric arterial beds from control (21.3 +/- 1.0 mmHg, n = 24) and pregnant (20.2 +/- 1.2 mmHg, n = 23) rats. However, a step-wise increase in perfusate flow from 5 to 10, 15, 20 and 24ml min-1 produced smaller increases in perfusion pressure in pregnancy compared to the controls. L-NAME (100 microM) or indomethacin (10 microM) had no significant effect on the relationship between flow and perfusion pressure. 8. The present results show that prejunctional changes are involved in blunted sympathetic vasoconstriction of rat mesenteric arteries in pregnancy. Non-specific postjunctional changes are implicated in the reduced constrictor responses to applied methoxamine, vasopressin and endothelin, but not to ATP. In contrast, sensory-motor nerves and endothelium-dependent and -independent vasodilatation was unchanged. The decrease in receptor-mediated mesenteric arterial constrictor responsiveness in pregnancy does not appear to be due to acute modulation by NO or prostaglandins, but may involve changes in the distensibility of the bed and/or changes in wall thickness.
Full text
PDF

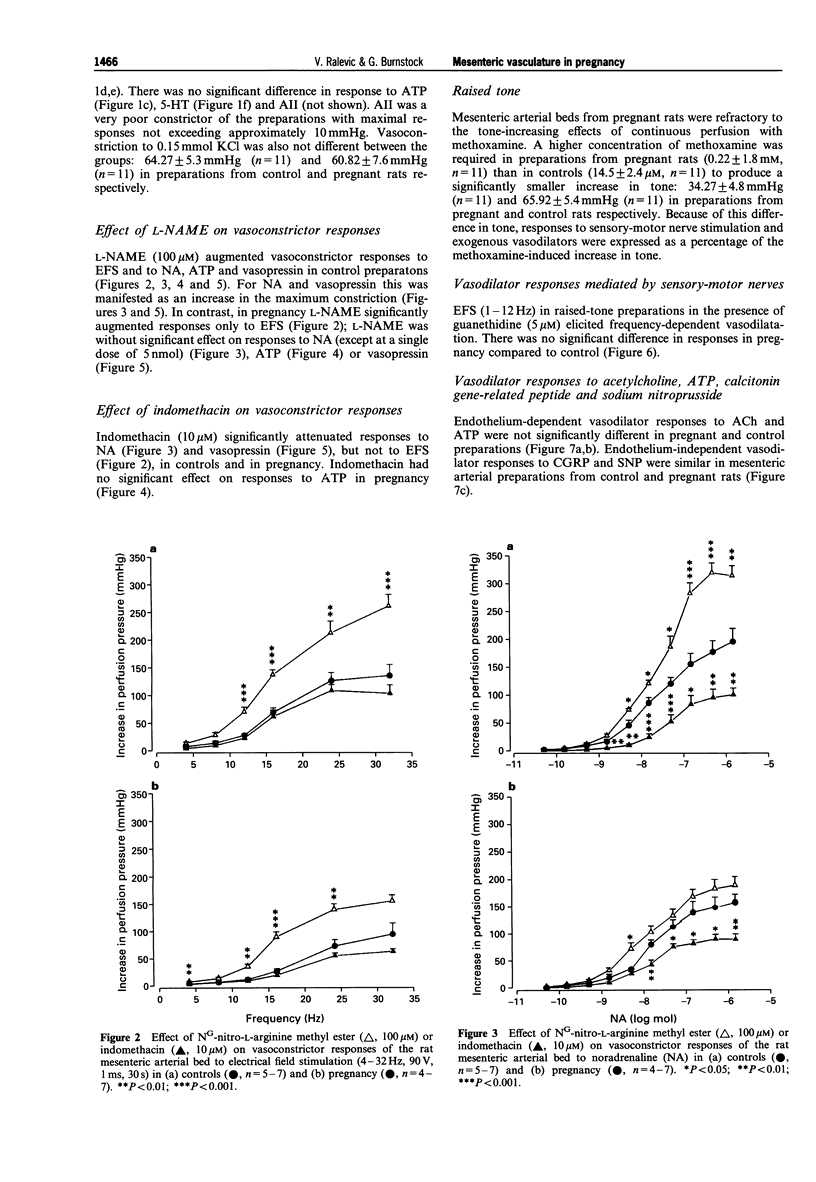
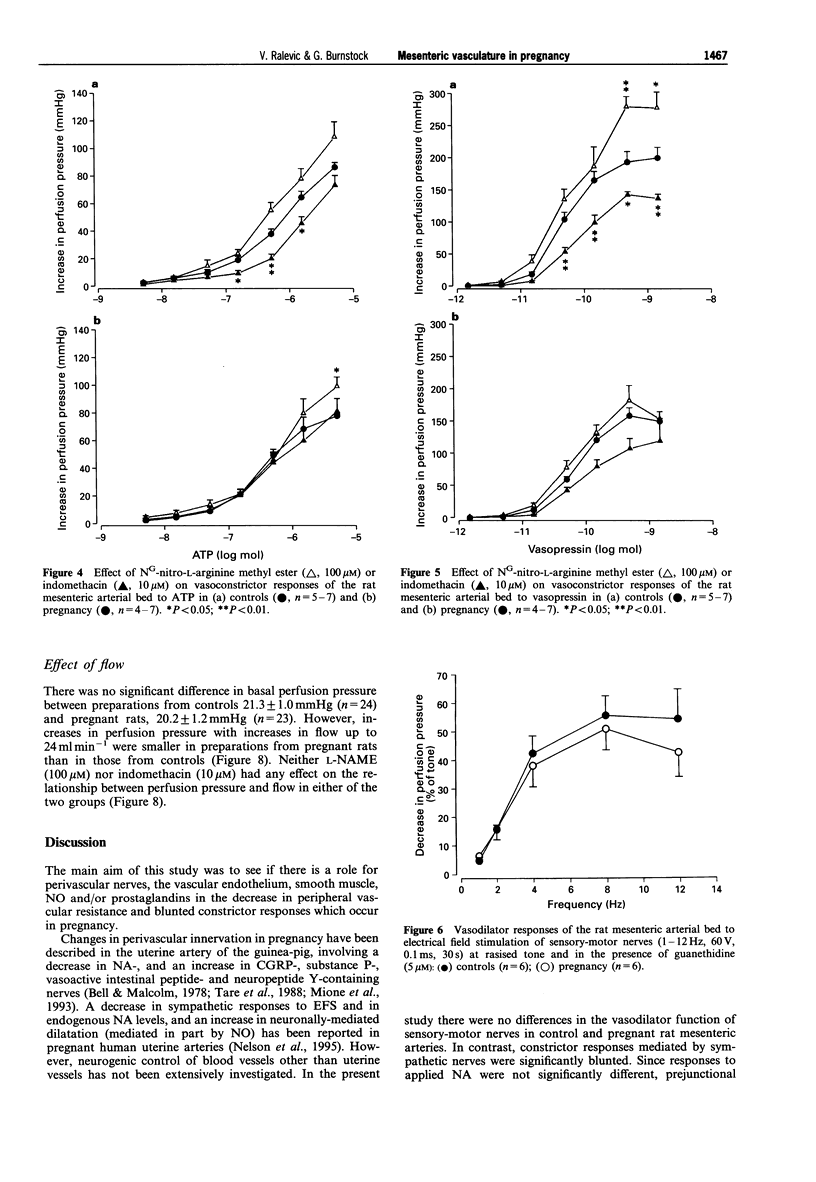


Selected References
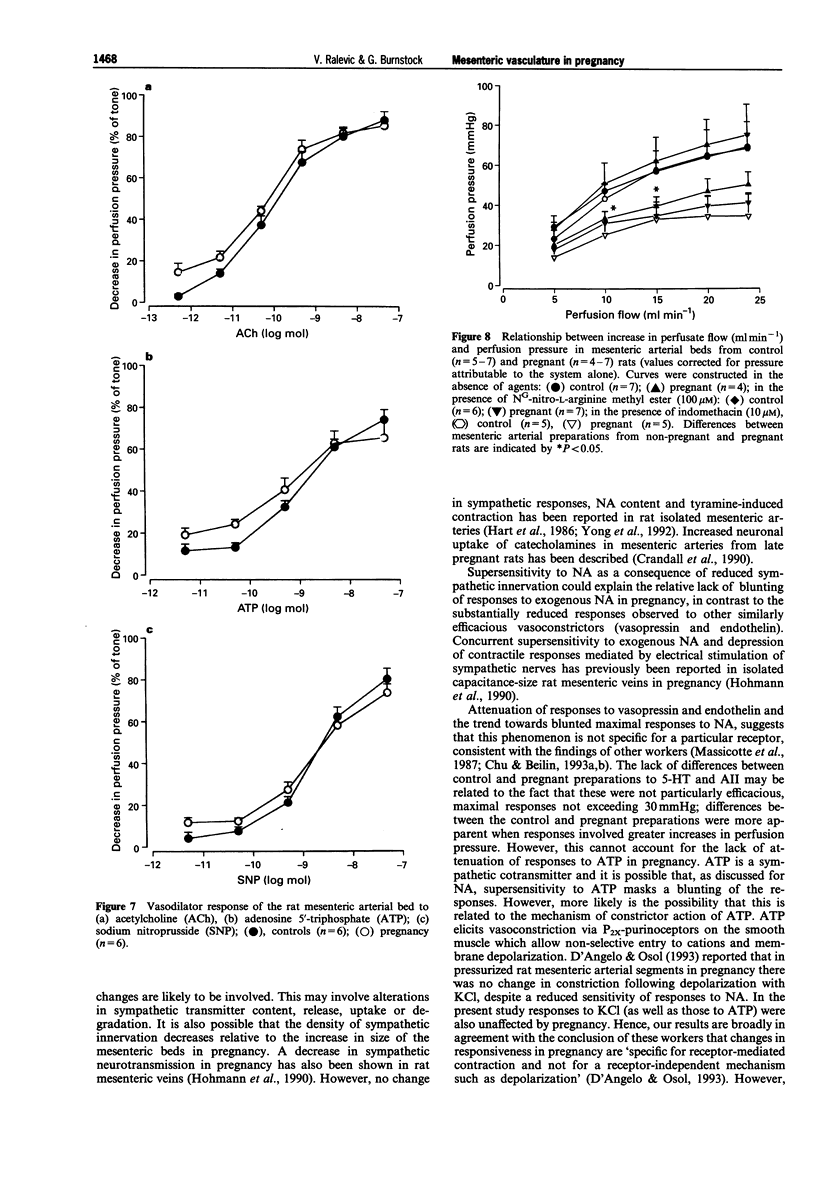
These references are in PubMed. This may not be the complete list of references from this article.
- Ahokas R. A., Mercer B. M., Sibai B. M. Enhanced endothelium-derived relaxing factor activity in pregnant, spontaneously hypertensive rats. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 1):801–807. doi: 10.1016/0002-9378(91)90420-v. [DOI] [PubMed] [Google Scholar]
- Bell C., Malcolm S. J. Observations on the loss of catecholamine fluorescence from intrauterine adrenergic nerves during pregnancy in the guinea-pig. J Reprod Fertil. 1978 May;53(1):51–58. doi: 10.1530/jrf.0.0530051. [DOI] [PubMed] [Google Scholar]
- Brown G. P., Venuto R. C. Angiotensin II receptor alterations during pregnancy in rabbits. Am J Physiol. 1986 Jul;251(1 Pt 1):E58–E64. doi: 10.1152/ajpendo.1986.251.1.E58. [DOI] [PubMed] [Google Scholar]
- Chaudhuri G., Barone P., Lianos E., Hurd M., Lele A., Venuto R. Uterine and peripheral blood concentrations of vasodilator prostaglandins in conscious pregnant rabbits. Am J Obstet Gynecol. 1982 Dec 1;144(7):760–767. doi: 10.1016/0002-9378(82)90348-9. [DOI] [PubMed] [Google Scholar]
- Chu Z. M., Beilin L. J. Mechanisms of vasodilatation in pregnancy: studies of the role of prostaglandins and nitric-oxide in changes of vascular reactivity in the in situ blood perfused mesentery of pregnant rats. Br J Pharmacol. 1993 Jun;109(2):322–329. doi: 10.1111/j.1476-5381.1993.tb13573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z. M., Beilin L. J. Nitric oxide-mediated changes in vascular reactivity in pregnancy in spontaneously hypertensive rats. Br J Pharmacol. 1993 Nov;110(3):1184–1188. doi: 10.1111/j.1476-5381.1993.tb13939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad K. P., Colpoys M. C. Evidence against the hypothesis that prostaglandins are the vasodepressor agents of pregnancy. Serial studies in chronically instrumented, conscious rats. J Clin Invest. 1986 Jan;77(1):236–245. doi: 10.1172/JCI112282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad K. P., Joffe G. M., Kruszyna H., Kruszyna R., Rochelle L. G., Smith R. P., Chavez J. E., Mosher M. D. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993 Apr 1;7(6):566–571. [PubMed] [Google Scholar]
- Conrad K. P., Vernier K. A. Plasma level, urinary excretion, and metabolic production of cGMP during gestation in rats. Am J Physiol. 1989 Oct;257(4 Pt 2):R847–R853. doi: 10.1152/ajpregu.1989.257.4.R847. [DOI] [PubMed] [Google Scholar]
- Crandall M. E., Keve T. M., McLaughlin M. K. Characterization of norepinephrine sensitivity in the maternal splanchnic circulation during pregnancy. Am J Obstet Gynecol. 1990 May;162(5):1296–1301. doi: 10.1016/0002-9378(90)90040-e. [DOI] [PubMed] [Google Scholar]
- D'Angelo G., Osol G. Regional variation in resistance artery diameter responses to alpha-adrenergic stimulation during pregnancy. Am J Physiol. 1993 Jan;264(1 Pt 2):H78–H85. doi: 10.1152/ajpheart.1993.264.1.H78. [DOI] [PubMed] [Google Scholar]
- Davidge S. T., McLaughlin M. K. Endogenous modulation of the blunted adrenergic response in resistance-sized mesenteric arteries from the pregnant rat. Am J Obstet Gynecol. 1992 Dec;167(6):1691–1698. doi: 10.1016/0002-9378(92)91763-z. [DOI] [PubMed] [Google Scholar]
- Dogterom J., De Jong W. Diminished pressor response to noradrenaline of the perfused tail artery of pregnant rats. Eur J Pharmacol. 1974 Feb;25(2):267–269. doi: 10.1016/0014-2999(74)90062-4. [DOI] [PubMed] [Google Scholar]
- Folkow B., Isaksson O. G., Karlström G., Lever A. F., Nordlander M. Trophic effects of hypophyseal hormones on resistance vessels and the heart in normotensive and renal hypertensive rats. Acta Physiol Scand. 1992 Mar;144(3):291–306. doi: 10.1111/j.1748-1716.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Gant N. F., Whalley P. J., Everett R. B., Worley R. J., MacDonald P. C. Control of vascular reactivity in pregnancy. Am J Kidney Dis. 1987 Apr;9(4):303–307. doi: 10.1016/s0272-6386(87)80126-9. [DOI] [PubMed] [Google Scholar]
- Goetz R. M., Morano I., Calovini T., Studer R., Holtz J. Increased expression of endothelial constitutive nitric oxide synthase in rat aorta during pregnancy. Biochem Biophys Res Commun. 1994 Nov 30;205(1):905–910. doi: 10.1006/bbrc.1994.2750. [DOI] [PubMed] [Google Scholar]
- Griggs K. C., Conrad K. P., Mackey K., McLaughlin M. K. Endothelial modulation of renal interlobar arteries from pregnant rats. Am J Physiol. 1993 Aug;265(2 Pt 2):F309–F315. doi: 10.1152/ajprenal.1993.265.2.F309. [DOI] [PubMed] [Google Scholar]
- Hart J. L., Freas W., Muldoon S. M. Neurovascular function in the rat during pregnancy. Am J Physiol. 1986 Nov;251(5 Pt 2):H1000–H1008. doi: 10.1152/ajpheart.1986.251.5.H1000. [DOI] [PubMed] [Google Scholar]
- Hohmann M., Keve T. M., Osol G., McLaughlin M. K. Norepinephrine sensitivity of mesenteric veins in pregnant rats. Am J Physiol. 1990 Oct;259(4 Pt 2):R753–R759. doi: 10.1152/ajpregu.1990.259.4.R753. [DOI] [PubMed] [Google Scholar]
- Hwa J. J., Ghibaudi L., Williams P., Chatterjee M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol. 1994 Mar;266(3 Pt 2):H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- Jansakul C., King R. G., Boura A. L. Effects of endothelial cell removal on alpha-adrenoceptor-mediated responses of aortae of pregnant rats. Clin Exp Pharmacol Physiol. 1990 Feb;17(2):147–156. doi: 10.1111/j.1440-1681.1990.tb01297.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Saito A., Takasaki K. Changes in calcitonin gene-related peptide (CGRP)-containing vasodilator nerve activity in hypertension. Brain Res. 1990 Jun 4;518(1-2):303–307. doi: 10.1016/0006-8993(90)90987-m. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Takasaki K., Saito A., Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988 Sep 8;335(6186):164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kim T. H., Weiner C. P., Thompson L. P. Effect of pregnancy on contraction and endothelium-mediated relaxation of renal and mesenteric arteries. Am J Physiol. 1994 Jul;267(1 Pt 2):H41–H47. doi: 10.1152/ajpheart.1994.267.1.H41. [DOI] [PubMed] [Google Scholar]
- Kondo K., Okuno T., Suzuki H., Saruta T. The effects of prostaglandins E2 and I2, and arachidonic acid on vascular reactivity to norepinephrine in isolated rat mesenteric artery, hind limb and splenic artery. Prostaglandins Med. 1980 Jan;4(1):21–30. doi: 10.1016/0161-4630(80)90060-9. [DOI] [PubMed] [Google Scholar]
- Li Y., Duckles S. P. Effect of age on vascular content of calcitonin gene-related peptide and mesenteric vasodilator nerve activity in the rat. Eur J Pharmacol. 1993 Jun 4;236(3):373–378. doi: 10.1016/0014-2999(93)90474-v. [DOI] [PubMed] [Google Scholar]
- MCGREGOR D. D. THE EFFECT OF SYMPATHETIC NERVE STIMULATION OF VASOCONSTRICTOR RESPONSES IN PERFUSED MESENTERIC BLOOD VESSELS OF THE RAT. J Physiol. 1965 Mar;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manku M. S., Horrobin D. F. Indomethacin inhibits responses to all vasoconstrictors in the rat mesenteric vascular bed: restoration of responses by prostaglandin E2. Prostaglandins. 1976 Sep;12(3):369–376. doi: 10.1016/0090-6980(76)90017-4. [DOI] [PubMed] [Google Scholar]
- Massicotte G., St-Louis J., Parent A., Schiffrin E. L. Decreased in vitro responses to vasoconstrictors during gestation in normotensive and spontaneously hypertensive rats. Can J Physiol Pharmacol. 1987 Dec;65(12):2466–2471. doi: 10.1139/y87-391. [DOI] [PubMed] [Google Scholar]
- McLaughlin M. K., Keve T. M. Pregnancy-induced changes in resistance blood vessels. Am J Obstet Gynecol. 1986 Dec;155(6):1296–1299. doi: 10.1016/0002-9378(86)90163-8. [DOI] [PubMed] [Google Scholar]
- Meyer M. C., Brayden J. E., McLaughlin M. K. Characteristics of vascular smooth muscle in the maternal resistance circulation during pregnancy in the rat. Am J Obstet Gynecol. 1993 Dec;169(6):1510–1516. doi: 10.1016/0002-9378(93)90427-k. [DOI] [PubMed] [Google Scholar]
- Mione M. C., Cavanagh J. F., Burnstock G. Uptake of 5-hydroxydopamine into non-sympathetic nerves of guinea-pig uterine artery in late pregnancy. J Neurocytol. 1993 Mar;22(3):164–175. doi: 10.1007/BF01246355. [DOI] [PubMed] [Google Scholar]
- Nelson S. H., Steinsland O. S., Johnson R. L., Suresh M. S., Gifford A., Ehardt J. S. Pregnancy-induced alterations of neurogenic constriction and dilation of human uterine artery. Am J Physiol. 1995 Apr;268(4 Pt 2):H1694–H1701. doi: 10.1152/ajpheart.1995.268.4.H1694. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Gregorini G., Ferris T. F. Pressor responsiveness in pseudopregnant and pregnant rats: role of maternal factors. Am J Physiol. 1989 Oct;257(4 Pt 2):R866–R871. doi: 10.1152/ajpregu.1989.257.4.R866. [DOI] [PubMed] [Google Scholar]
- Paller M. S. Mechanism of decreased pressor responsiveness to ANG II, NE, and vasopressin in pregnant rats. Am J Physiol. 1984 Jul;247(1 Pt 2):H100–H108. doi: 10.1152/ajpheart.1984.247.1.H100. [DOI] [PubMed] [Google Scholar]
- Parent A., Schiffrin E. L., St-Louis J. Receptors for Arg8-vasopressin, angiotensin II, and atrial natriuretic peptide in the mesenteric vasculature of pregnant rats. Can J Physiol Pharmacol. 1991 Feb;69(2):137–144. doi: 10.1139/y91-020. [DOI] [PubMed] [Google Scholar]
- Parent A., Schiffrin E. L., St-Louis J. Role of the endothelium in adrenergic responses of mesenteric artery rings of pregnant rats. Am J Obstet Gynecol. 1990 Jul;163(1 Pt 1):229–234. doi: 10.1016/s0002-9378(11)90703-0. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Belai A., Burnstock G. Impaired sensory-motor nerve function in the isolated mesenteric arterial bed of streptozotocin-diabetic and ganglioside-treated streptozotocin-diabetic rats. Br J Pharmacol. 1993 Nov;110(3):1105–1111. doi: 10.1111/j.1476-5381.1993.tb13928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron G. J., Garland C. J. Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br J Pharmacol. 1994 Jul;112(3):831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner C. P., Knowles R. G., Moncada S. Induction of nitric oxide synthases early in pregnancy. Am J Obstet Gynecol. 1994 Sep;171(3):838–843. doi: 10.1016/0002-9378(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Weiner C. P., Lizasoain I., Baylis S. A., Knowles R. G., Charles I. G., Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner C., Martinez E., Zhu L. K., Ghodsi A., Chestnut D. In vitro release of endothelium-derived relaxing factor by acetylcholine is increased during the guinea pig pregnancy. Am J Obstet Gynecol. 1989 Dec;161(6 Pt 1):1599–1605. doi: 10.1016/0002-9378(89)90933-2. [DOI] [PubMed] [Google Scholar]
- Yong E. M., Mano M. T., Head R. J. Neurovascular function during pregnancy in the spontaneously hypertensive rat. Clin Exp Pharmacol Physiol. 1992 Jun;19(6):415–423. doi: 10.1111/j.1440-1681.1992.tb00484.x. [DOI] [PubMed] [Google Scholar]


