Abstract
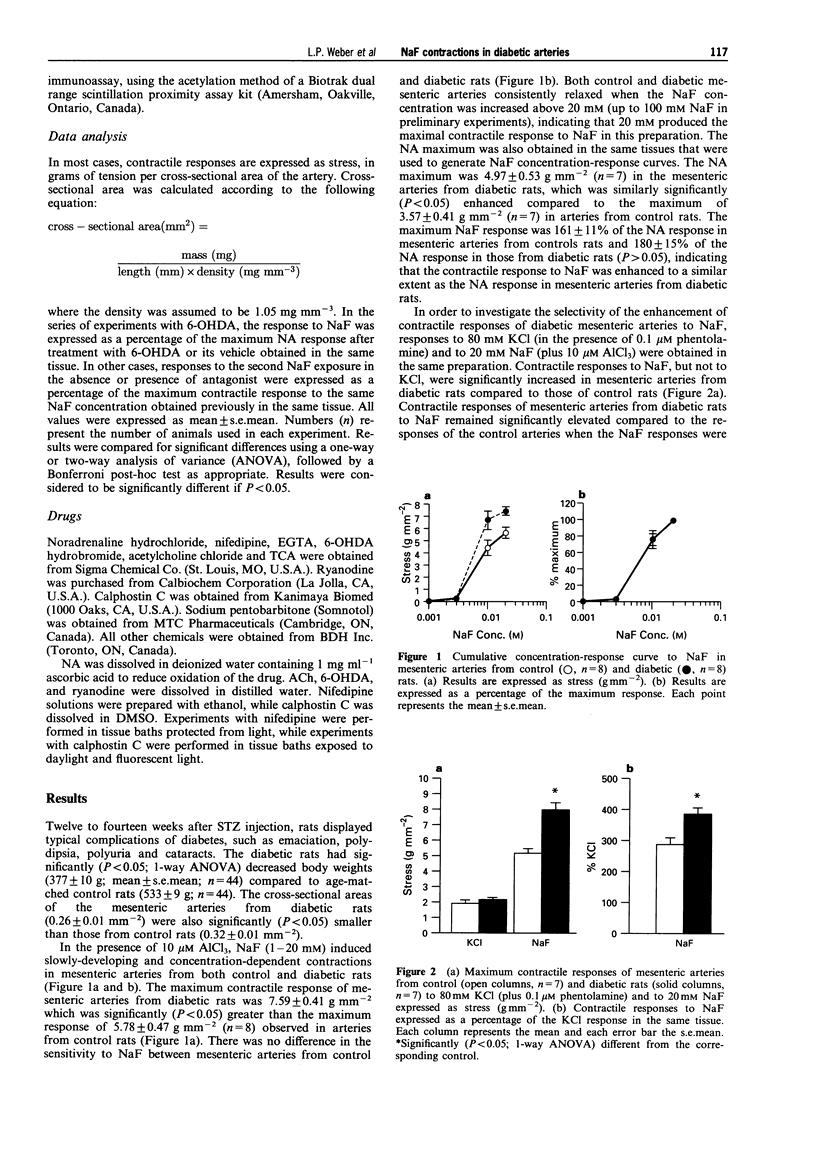
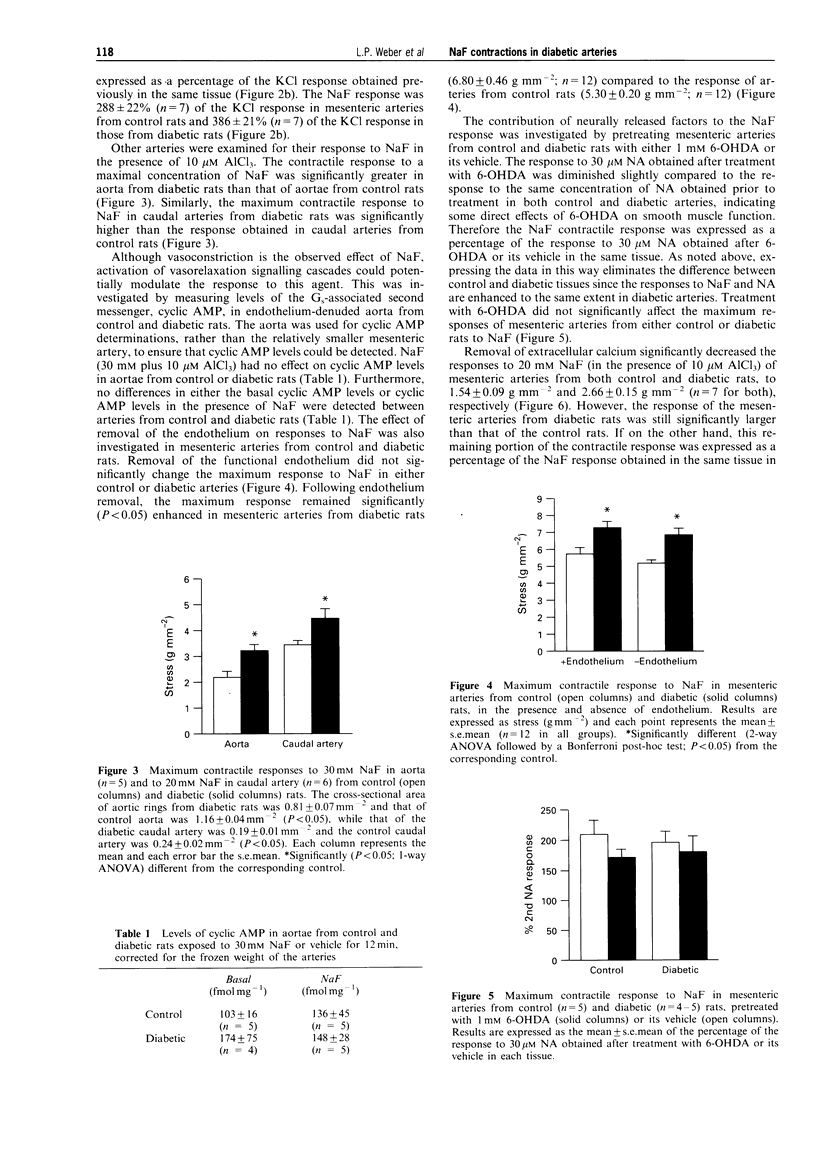
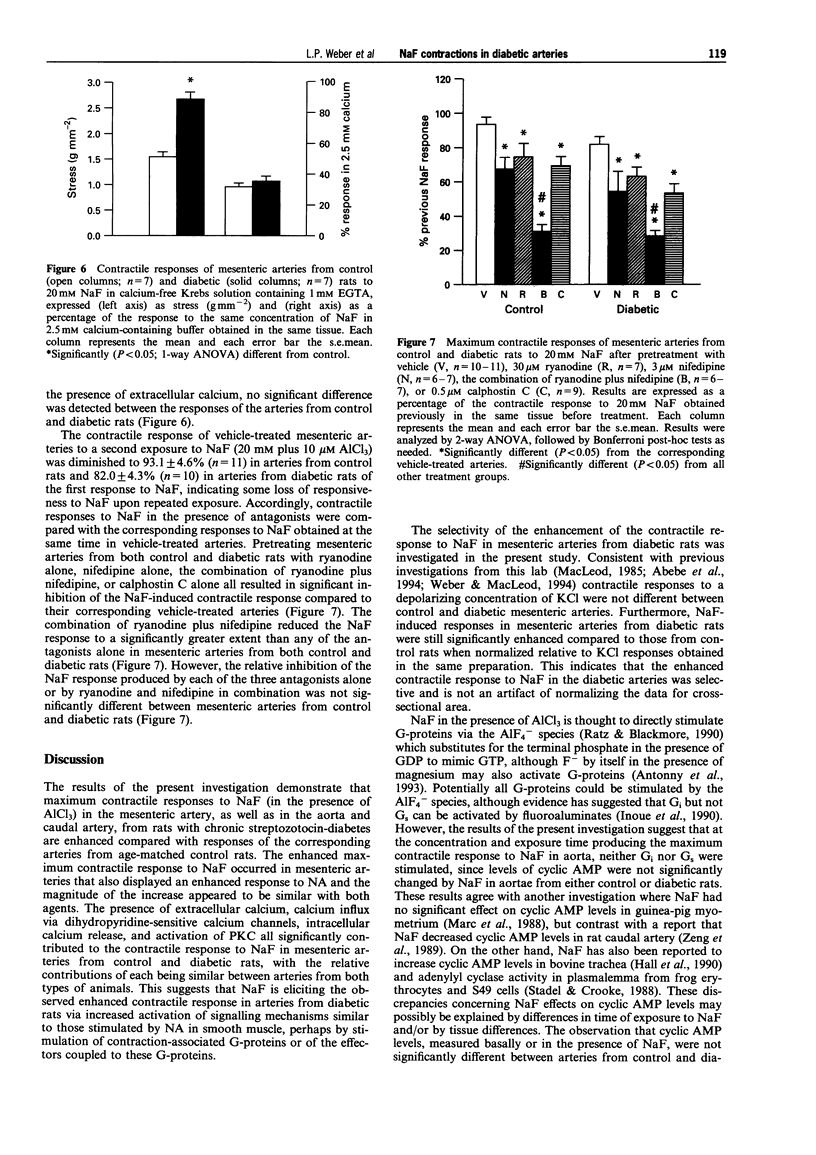
1. Previous studies from this laboratory have demonstrated that alpha 1-adrenoceptor-mediated increases in tension and phosphoinositide metabolism are enhanced in the aorta and mesenteric arteries from diabetic rats. The purpose of the present investigation was to determine whether contractile responses to sodium fluoride (NaF), which directly stimulates GTP-binding proteins (G-proteins), are also enhanced in diabetic arteries. 2. NaF (1-20 mM) in the presence of 10 microM aluminium chloride produced slowly developing, concentration-dependent contractions in mesenteric arteries from three month streptozotocin-diabetic (60 mg kg-1, i.v.) male Wistar rats and age-matched control rats. The maximum contractile response but not the sensitivity to NaF was significantly greater in mesenteric arteries from diabetic than from control rats, as was the response to noradrenaline (NA). Maximum contractile responses of aorta and caudal artery from diabetic rats to NaF were also significantly enhanced. 3. Removal of the endothelium and denervation with 6-hydroxydopamine did not significantly alter the maximum contractile response of mesenteric arteries from either control or diabetic rats to NaF. Similarly, NaF had no effect on cyclic AMP levels in aorta, and no difference in cyclic AMP levels, either basally or in the presence of NaF, was detected between control and diabetic rat aorta. 4. Contractile responses of mesenteric arteries from both control and diabetic rats to NaF were diminished in calcium-free Krebs solution, but the NaF response remained significantly elevated in mesenteric arteries from diabetic rats compared to control. 5. Ryanodine (30 microM) which depletes intracellular calcium stores, nifedipine (3 microM) which blocks dihydropyridine-sensitive calcium channels and calphostin C (0.5 microM) which selectively inhibits protein kinase C, all significantly inhibited maximum contractile responses of mesenteric arteries from control and diabetic rats to NaF. There were no significant differences between control and diabetic arteries in the relative magnitude of the inhibition produce by the three antagonist. 6. These data suggest that there may be increased activation of the same signalling processes that mediate NA-stimulated vasoconstriction, perhaps contraction-associated G-proteins or the effectors coupled to these G-proteins, in response to NaF in mesenteric arteries from diabetic rats. This may also be responsible for the enhanced contractile responses of these arteries to alpha 1-adrenoceptor stimulation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Abebe W., Edwards J. D., Agrawal D. K. G-proteins in rat blood vessels--II. Assessment of functional involvement. Gen Pharmacol. 1995 Jan;26(1):75–83. doi: 10.1016/0306-3623(94)00174-l. [DOI] [PubMed] [Google Scholar]
- Abebe W., Harris K. H., MacLeod K. M. Enhanced contractile responses of arteries from diabetic rats to alpha 1-adrenoceptor stimulation in the absence and presence of extracellular calcium. J Cardiovasc Pharmacol. 1990 Aug;16(2):239–248. doi: 10.1097/00005344-199008000-00010. [DOI] [PubMed] [Google Scholar]
- Abebe W., Harris K. H., MacLeod K. M. Role of extracellular Ca2+ in the selective enhancement of contractile responses of arteries from diabetic rats to noradrenaline. Can J Physiol Pharmacol. 1994 Dec;72(12):1544–1551. doi: 10.1139/y94-222. [DOI] [PubMed] [Google Scholar]
- Abebe W., MacLeod K. M. Augmented inositol phosphate production in mesenteric arteries from diabetic rats. Eur J Pharmacol. 1992 Jan 14;225(1):29–36. doi: 10.1016/0922-4106(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Abebe W., MacLeod K. M. Enhanced arterial contractility to noradrenaline in diabetic rats is associated with increased phosphoinositide metabolism. Can J Physiol Pharmacol. 1991 Mar;69(3):355–361. doi: 10.1139/y91-054. [DOI] [PubMed] [Google Scholar]
- Abebe W., MacLeod K. M. Influence of diabetes on norepinephrine-induced inositol 1,4,5-trisphosphate levels in rat aorta. Life Sci. 1991;49(13):PL85–PL90. doi: 10.1016/0024-3205(91)90084-o. [DOI] [PubMed] [Google Scholar]
- Abebe W., MacLeod K. M. Protein kinase C-mediated contractile responses of arteries from diabetic rats. Br J Pharmacol. 1990 Oct;101(2):465–471. doi: 10.1111/j.1476-5381.1990.tb12731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeagbo A. S., Triggle C. R. Mechanism of vascular smooth muscle contraction by sodium fluoride in the isolated aorta of rat and rabbit. J Pharmacol Exp Ther. 1991 Jul 1;258(1):66–73. [PubMed] [Google Scholar]
- Agrawal D. K., McNeill J. H. Effect of diabetes on vascular smooth muscle function in normotensive and spontaneously hypertensive rat mesenteric artery. Can J Physiol Pharmacol. 1987 Nov;65(11):2274–2280. doi: 10.1139/y87-360. [DOI] [PubMed] [Google Scholar]
- Antonny B., Sukumar M., Bigay J., Chabre M., Higashijima T. The mechanism of aluminum-independent G-protein activation by fluoride and magnesium. 31P NMR spectroscopy and fluorescence kinetic studies. J Biol Chem. 1993 Feb 5;268(4):2393–2402. [PubMed] [Google Scholar]
- Berta P., Phaneuf S., Travo P., Cavadore J. C. Modulation of the accumulation of inositol phosphates and the mobilization of calcium in aortic myocytes. Eur J Pharmacol. 1988 Aug 9;153(1):123–129. doi: 10.1016/0014-2999(88)90596-1. [DOI] [PubMed] [Google Scholar]
- Bollen M., Stalmans W. Fluorine compounds inhibit the conversion of active type-1 protein phosphatases into the ATPMg-dependent form. Biochem J. 1988 Oct 1;255(1):327–333. [PMC free article] [PubMed] [Google Scholar]
- Boonen H. C., De Mey J. G. G-proteins are involved in contractile responses of isolated mesenteric resistance arteries to agonists. Naunyn Schmiedebergs Arch Pharmacol. 1990 Oct;342(4):462–468. doi: 10.1007/BF00169465. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Miller F. D., Merriman R. L., Howbert J. J., Heath W. F., Kobayashi E., Takahashi I., Tamaoki T., Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991 Apr 15;176(1):288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Cheung Y. D., Feltham I., Thompson P., Triggle C. R. Alpha-adrenoceptor activation of polyphosphoinositide hydrolysis in the rat tail artery. Biochem Pharmacol. 1990 Dec 1;40(11):2425–2432. doi: 10.1016/0006-2952(90)90082-v. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., McCall D. E., Thoolen M. J., Timmermans P. B. Ca++ utilization in the constriction of rat aorta to full and partial alpha-1 adrenoceptor agonists. J Pharmacol Exp Ther. 1986 Jul;238(1):224–231. [PubMed] [Google Scholar]
- Christlieb A. R., Janka H. U., Kraus B., Gleason R. E., Icasas-Cabral E. A., Aiello L. M., Cabral B. V., Solano A. Vascular reactivity to angiotensin II and to norepinephrine in diabetic subjects. Diabetes. 1976 Apr;25(4):268–274. doi: 10.2337/diab.25.4.268. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Tesfamariam B., Weisbrod R. M., Zitnay K. M. Adrenergic denervation in rabbits with diabetes mellitus. Am J Physiol. 1990 Jul;259(1 Pt 2):H55–H61. doi: 10.1152/ajpheart.1990.259.1.H55. [DOI] [PubMed] [Google Scholar]
- Cushing D. J., Sabouni M. H., Brown G. L., Mustafa S. J. Fluoride produces endothelium-dependent relaxation and endothelium-independent contraction in coronary artery. J Pharmacol Exp Ther. 1990 Jul;254(1):28–32. [PubMed] [Google Scholar]
- Godfraind T. Actions of nifedipine on calcium fluxes and contraction in isolated rat arteries. J Pharmacol Exp Ther. 1983 Feb;224(2):443–450. [PubMed] [Google Scholar]
- Hall I. P., Donaldson J., Hill S. J. Modulation of fluoroaluminate-induced inositol phosphate formation by increases in tissue cyclic AMP content in bovine tracheal smooth muscle. Br J Pharmacol. 1990 Jul;100(3):646–650. doi: 10.1111/j.1476-5381.1990.tb15861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. H., MacLeod K. M. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur J Pharmacol. 1988 Aug 9;153(1):55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- Hart J. L., Freas W., McKenzie J. E., Muldoon S. M. Adrenergic nerve function and contractile activity of the caudal artery of the streptozotocin diabetic rat. J Auton Nerv Syst. 1988 Nov;25(1):49–57. doi: 10.1016/0165-1838(88)90007-0. [DOI] [PubMed] [Google Scholar]
- Henrion D., Laher I. Effects of staurosporine and calphostin C, two structurally unrelated inhibitors of protein kinase C, on vascular tone. Can J Physiol Pharmacol. 1993 Jul;71(7):521–524. doi: 10.1139/y93-076. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Fishman P. H., Rebois R. V. Differential activation of the stimulatory and inhibitory guanine nucleotide-binding proteins by fluoroaluminate in cells and in membranes. J Biol Chem. 1990 Jun 25;265(18):10645–10651. [PubMed] [Google Scholar]
- MacLeod K. M. The effect of insulin treatment on changes in vascular reactivity in chronic, experimental diabetes. Diabetes. 1985 Nov;34(11):1160–1167. doi: 10.2337/diab.34.11.1160. [DOI] [PubMed] [Google Scholar]
- Marc S., Leiber D., Harbon S. Fluoroaluminates mimic muscarinic- and oxytocin-receptor-mediated generation of inositol phosphates and contraction in the intact guinea-pig myometrium. Role for a pertussis/cholera-toxin-insensitive G protein. Biochem J. 1988 Oct 15;255(2):705–713. [PMC free article] [PubMed] [Google Scholar]
- Murphy A. J., Coll R. J. Fluoride is a slow, tight-binding inhibitor of the calcium ATPase of sarcoplasmic reticulum. J Biol Chem. 1992 Mar 15;267(8):5229–5235. [PubMed] [Google Scholar]
- Murphy A. J., Hoover J. C. Inhibition of the Na,K-ATPase by fluoride. Parallels with its inhibition of the sarcoplasmic reticulum CaATPase. J Biol Chem. 1992 Aug 25;267(24):16995–16700. [PubMed] [Google Scholar]
- Murthy K. S., Makhlouf G. M. Fluoride activates G protein-dependent and -independent pathways in dispersed intestinal smooth muscle cells. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1681–1687. doi: 10.1006/bbrc.1994.2128. [DOI] [PubMed] [Google Scholar]
- Nichols A. J., Motley E. D., Ruffolo R. R., Jr Effect of pertussis toxin treatment on postjunctional alpha-1 and alpha-2 adrenoceptor function in the cardiovascular system of the pithed rat. J Pharmacol Exp Ther. 1989 Apr;249(1):203–209. [PubMed] [Google Scholar]
- Oyama Y., Kawasaki H., Hattori Y., Kanno M. Attenuation of endothelium-dependent relaxation in aorta from diabetic rats. Eur J Pharmacol. 1986 Dec 2;132(1):75–78. doi: 10.1016/0014-2999(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Pfaffman M. A., Ball C. R., Darby A., Hilman R. Insulin reversal of diabetes-induced inhibition of vascular contractility in the rat. Am J Physiol. 1982 Apr;242(4):H490–H495. doi: 10.1152/ajpheart.1982.242.4.H490. [DOI] [PubMed] [Google Scholar]
- Ramanadham S., Lyness W. H., Tenner T. E., Jr Alterations in aortic and tail artery reactivity to agonists after streptozotocin treatment. Can J Physiol Pharmacol. 1984 Apr;62(4):418–423. doi: 10.1139/y84-066. [DOI] [PubMed] [Google Scholar]
- Ratz P. H., Blackmore P. F. Differential activation of rabbit femoral arteries by aluminum fluoride and sodium fluoride. J Pharmacol Exp Ther. 1990 Aug;254(2):514–520. [PubMed] [Google Scholar]
- Shimamoto Y., Shimamoto H., Kwan C. Y., Daniel E. E. Differential effects of putative protein kinase C inhibitors on contraction of rat aortic smooth muscle. Am J Physiol. 1993 Apr;264(4 Pt 2):H1300–H1306. doi: 10.1152/ajpheart.1993.264.4.H1300. [DOI] [PubMed] [Google Scholar]
- Stadel J. M., Crooke S. T. Differential effects of fluoride on adenylate cyclase activity and guanine nucleotide regulation of agonist high-affinity receptor binding. Biochem J. 1988 Aug 15;254(1):15–20. doi: 10.1042/bj2540015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi Y., Satoh N., Hashimoto H., Nakashima M. Reversal effect of thyroxine on altered vascular reactivity in diabetic rats. J Cardiovasc Pharmacol. 1989 Apr;13(4):520–524. [PubMed] [Google Scholar]
- Taylor P. D., Wickenden A. D., Mirrlees D. J., Poston L. Endothelial function in the isolated perfused mesentery and aortae of rats with streptozotocin-induced diabetes: effect of treatment with the aldose reductase inhibitor, ponalrestat. Br J Pharmacol. 1994 Jan;111(1):42–48. doi: 10.1111/j.1476-5381.1994.tb14021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiger G., Björklund P. E., Brännström G., Fowler C. J. Multiple actions of fluoride ions upon the phosphoinositide cycle in the rat brain. Brain Res. 1990 Dec 24;537(1-2):93–101. doi: 10.1016/0006-8993(90)90344-b. [DOI] [PubMed] [Google Scholar]
- Weber L. P., Chow W. L., Moshenko J., Belsher S., MacLeod K. M. Pharmacological investigation of signaling mechanisms contributing to phasic and tonic components of the contractile response of rat arteries to noradrenaline. Can J Physiol Pharmacol. 1995 May;73(5):594–601. doi: 10.1139/y95-075. [DOI] [PubMed] [Google Scholar]
- Weber L. P., MacLeod K. M. Contractile responses of caudal arteries from diabetic rats to adrenergic nerve stimulation. J Vasc Res. 1994 Jan-Feb;31(1):25–32. doi: 10.1159/000159028. [DOI] [PubMed] [Google Scholar]
- White R. E., Carrier G. O. Enhanced vascular alpha-adrenergic neuroeffector system in diabetes: importance of calcium. Am J Physiol. 1988 Nov;255(5 Pt 2):H1036–H1042. doi: 10.1152/ajpheart.1988.255.5.H1036. [DOI] [PubMed] [Google Scholar]
- Zeng Y. Y., Benishin C. G., Pang P. K. Guanine nucleotide binding proteins may modulate gating of calcium channels in vascular smooth muscle. I. Studies with fluoride. J Pharmacol Exp Ther. 1989 Jul;250(1):343–351. [PubMed] [Google Scholar]