Abstract
1. Angiotensin II (AII) actions are mediated by two distinct types of receptors: AT1, which includes two subtypes, AT1A and AT1B, and AT2. AII produces vasoconstriction on the vascular wall acting directly on smooth muscle cells via AT1 receptors. AII receptors have recently been demonstrated on endothelial cells. But the pharmacological characteristics of these receptors and the intracellular signal pathways coupled to them remain unclear. 2. The aim of this work was to characterize the AII receptor subtypes in rat aortic endothelial cells (RAEC) in primary culture and to evaluate the signal pathways coupled to these receptors by measuring the activation of phospholipase C (PLC) and phospholipase A2 (PLA2). 3. Labelled AII bound to RAEC in a specific, saturable manner. Scatchard analysis showed a Kd of 1.87 +/- 0.49 nM and a Bmax of 50.2 +/- 10.9 x 10(3) sites per cell. AII was displaced by the AT1-specific antagonist, DuP753 with a Ki of 17.37 +/- 1.49 nM, but not by the AT2 receptor analogues CGP42771B or PD123177. These data were confirmed by the finding of AT1 mRNA in endothelial cells. Analysis of RNA expression by RT-PCR showed the presence of both subtypes, AT1A and AT1B in endothelial cells, whereas smooth muscle cells express only AT1A. 4. The activation of PLC and PLA2 in response to AII was evaluated by measuring inositol phosphate production and arachidonic acid release, respectively. Both were enhanced by AII in a dose-dependent manner, and inhibited by DuP753, but not by PD123177. 5. We conclude that AT1 receptors are expressed by endothelial cells in primary culture and that phospholipase C and phospholipase A2 activated via this receptor.
Full text
PDF

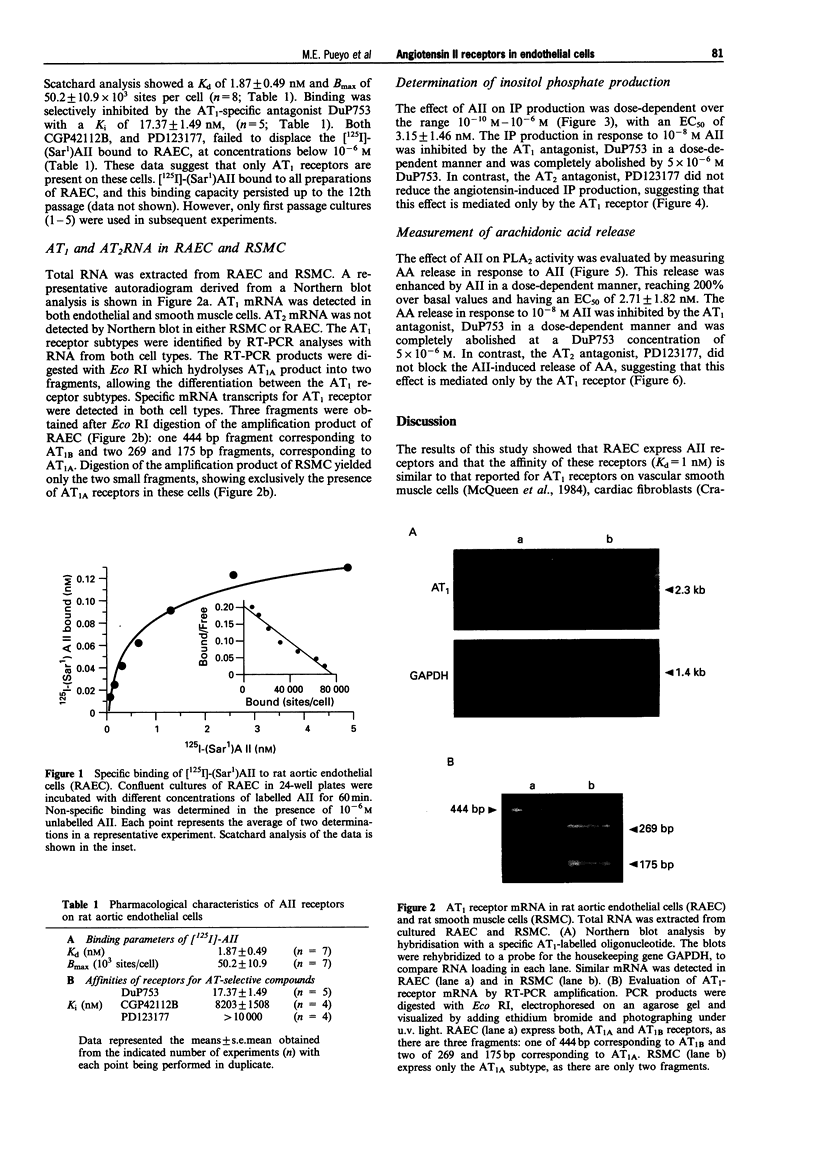
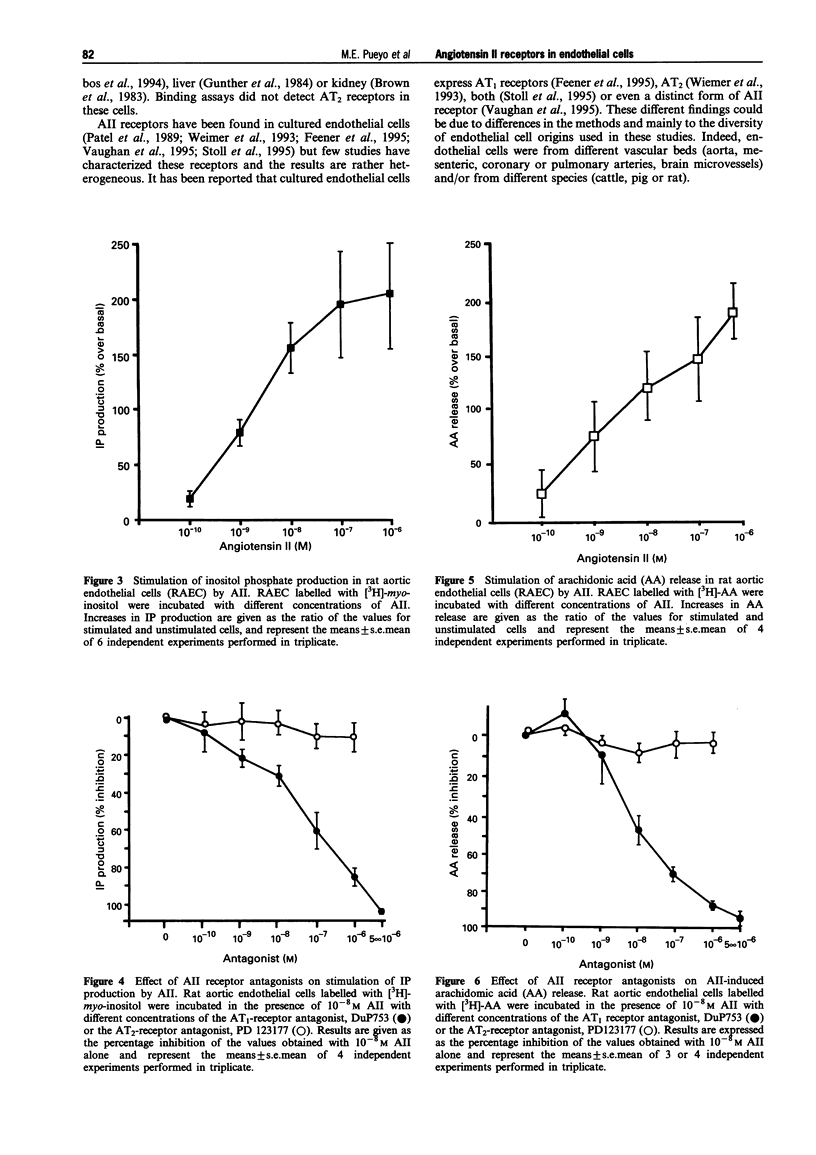


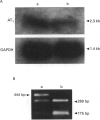
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battle T., Arnal J. F., Challah M., Michel J. B. Selective isolation of rat aortic wall layers and their cell types in culture--application to converting enzyme activity measurement. Tissue Cell. 1994 Dec;26(6):943–955. doi: 10.1016/0040-8166(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Bottari S. P., de Gasparo M., Steckelings U. M., Levens N. R. Angiotensin II receptor subtypes: characterization, signalling mechanisms, and possible physiological implications. Front Neuroendocrinol. 1993 Apr;14(2):123–171. doi: 10.1006/frne.1993.1005. [DOI] [PubMed] [Google Scholar]
- Brown G. P., Douglas J. G. Angiotensin II-binding sites in rat and primate isolated renal tubular basolateral membranes. Endocrinology. 1983 Jun;112(6):2007–2014. doi: 10.1210/endo-112-6-2007. [DOI] [PubMed] [Google Scholar]
- Buckley B. J., Barchowsky A., Dolor R. J., Whorton A. R. Regulation of arachidonic acid release in vascular endothelium. Ca(2+)-dependent and -independent pathways. Biochem J. 1991 Dec 1;280(Pt 2):281–287. doi: 10.1042/bj2800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo L., Benessiano J., Boulanger C. M., Lévy B. I. Angiotensin II increases cGMP content via endothelial angiotensin II AT1 subtype receptors in the rat carotid artery. Arterioscler Thromb Vasc Biol. 1995 Oct;15(10):1646–1651. doi: 10.1161/01.atv.15.10.1646. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., Dunscomb J., Kosierowski J., Burton C. R., Santomenna L. D., Corjay M. H., Benfield P. The ligand binding signatures of the rat AT1A, AT1B and the human AT1 receptors are essentially identical. Biochem Biophys Res Commun. 1993 Dec 15;197(2):440–449. doi: 10.1006/bbrc.1993.2499. [DOI] [PubMed] [Google Scholar]
- Chiu A. T., McCall D. E., Price W. A., Wong P. C., Carini D. J., Duncia J. V., Wexler R. R., Yoo S. E., Johnson A. L., Timmermans P. B. Nonpeptide angiotensin II receptor antagonists. VII. Cellular and biochemical pharmacology of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990 Feb;252(2):711–718. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crabos M., Roth M., Hahn A. W., Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts. Coupling to signaling systems and gene expression. J Clin Invest. 1994 Jun;93(6):2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danser A. H., Chowdury S., de Lannoy L. M., van der Giessen W. J., Saxena P. R., Schalekamp M. A. Conversion and degradation of [125I] labelled angiotensin I in isolated perfused porcine coronary and carotid arteries. Cardiovasc Res. 1995 Jun;29(6):789–795. [PubMed] [Google Scholar]
- Dostal D. E., Murahashi T., Peach M. J. Regulation of cytosolic calcium by angiotensins in vascular smooth muscle. Hypertension. 1990 Jun;15(6 Pt 2):815–822. doi: 10.1161/01.hyp.15.6.815. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., van Goor H., Klatter F., Majoor G. D., van Bussel E., van Breda Vriesman P. J. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992 Apr;66(4):459–466. [PubMed] [Google Scholar]
- Feener E. P., Northrup J. M., Aiello L. P., King G. L. Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J Clin Invest. 1995 Mar;95(3):1353–1362. doi: 10.1172/JCI117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. A., Gross R. W. Plasmenylethanolamine is the major storage depot for arachidonic acid in rabbit vascular smooth muscle and is rapidly hydrolyzed after angiotensin II stimulation. Proc Natl Acad Sci U S A. 1989 May;86(10):3479–3483. doi: 10.1073/pnas.86.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasc J. M., Shanmugam S., Sibony M., Corvol P. Tissue-specific expression of type 1 angiotensin II receptor subtypes. An in situ hybridization study. Hypertension. 1994 Nov;24(5):531–537. doi: 10.1161/01.hyp.24.5.531. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Alexander R. W. Angiotensin II stimulation of prostaglandin production in cultured human vascular endothelium. Science. 1975 Jul 18;189(4198):219–220. doi: 10.1126/science.1138377. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Ryan E. T., Lemke S. M., Bailly D. A., Fox M. K., Schoepp D. D. Endothelium-dependent modulation of angiotensin II-induced contraction in blood vessels. Eur J Pharmacol. 1988 Jan 27;146(1):85–95. doi: 10.1016/0014-2999(88)90489-x. [DOI] [PubMed] [Google Scholar]
- Gunther S. Characterization of angiotensin II receptor subtypes in rat liver. J Biol Chem. 1984 Jun 25;259(12):7622–7629. [PubMed] [Google Scholar]
- Kambayashi Y., Bardhan S., Inagami T. Peptide growth factors markedly decrease the ligand binding of angiotensin II type 2 receptor in rat cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Jul 15;194(1):478–482. doi: 10.1006/bbrc.1993.1844. [DOI] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Lewis J. L., Serikawa T., Warnock D. G. Chromosomal localization of angiotensin II type 1 receptor isoforms in the rat. Biochem Biophys Res Commun. 1993 Jul 30;194(2):677–682. doi: 10.1006/bbrc.1993.1875. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Cortes C., Greenberg B., Huang H., Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension. 1994 Nov;24(5):538–548. doi: 10.1161/01.hyp.24.5.538. [DOI] [PubMed] [Google Scholar]
- Lokuta A. J., Cooper C., Gaa S. T., Wang H. E., Rogers T. B. Angiotensin II stimulates the release of phospholipid-derived second messengers through multiple receptor subtypes in heart cells. J Biol Chem. 1994 Feb 18;269(7):4832–4838. [PubMed] [Google Scholar]
- Marrero M. B., Paxton W. G., Duff J. L., Berk B. C., Bernstein K. E. Angiotensin II stimulates tyrosine phosphorylation of phospholipase C-gamma 1 in vascular smooth muscle cells. J Biol Chem. 1994 Apr 8;269(14):10935–10939. [PubMed] [Google Scholar]
- McQueen J., Murray G. D., Semple P. F. Identification of the angiotensin II receptor in rat mesenteric artery. Biochem J. 1984 Nov 1;223(3):659–671. doi: 10.1042/bj2230659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Peach M. J. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977 Apr;57(2):313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Signal transduction pathways of angiotensin II--induced c-fos gene expression in cardiac myocytes in vitro. Roles of phospholipid-derived second messengers. Circ Res. 1993 Sep;73(3):424–438. doi: 10.1161/01.res.73.3.424. [DOI] [PubMed] [Google Scholar]
- Shanmugam S., Lenkei Z. G., Gasc J. M., Corvol P. L., Llorens-Cortes C. M. Ontogeny of angiotensin II type 2 (AT2) receptor mRNA in the rat. Kidney Int. 1995 Apr;47(4):1095–1100. doi: 10.1038/ki.1995.156. [DOI] [PubMed] [Google Scholar]
- Stoll M., Steckelings U. M., Paul M., Bottari S. P., Metzger R., Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995 Feb;95(2):651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F., Benfield P., Carini D. J., Lee R. J., Wexler R. R., Saye J. A., Smith R. D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- Vaughan D. E., Lazos S. A., Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995 Mar;95(3):995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M., Tsutsumi K., Correa F. M., Saavedra J. M. Changes in expression of angiotensin receptor subtypes in the rat aorta during development. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1361–1367. doi: 10.1016/0006-291x(91)91723-p. [DOI] [PubMed] [Google Scholar]
- Whitebread S., Mele M., Kamber B., de Gasparo M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem Biophys Res Commun. 1989 Aug 30;163(1):284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- Zhang J., Van Meel J. C., Pfaffendorf M., Zhang J., Van Zwieten P. A. Endothelium-dependent, nitric oxide-mediated inhibition of angiotensin II-induced contractions in rabbit aorta. Eur J Pharmacol. 1994 Sep 12;262(3):247–253. doi: 10.1016/0014-2999(94)90738-2. [DOI] [PubMed] [Google Scholar]