Abstract
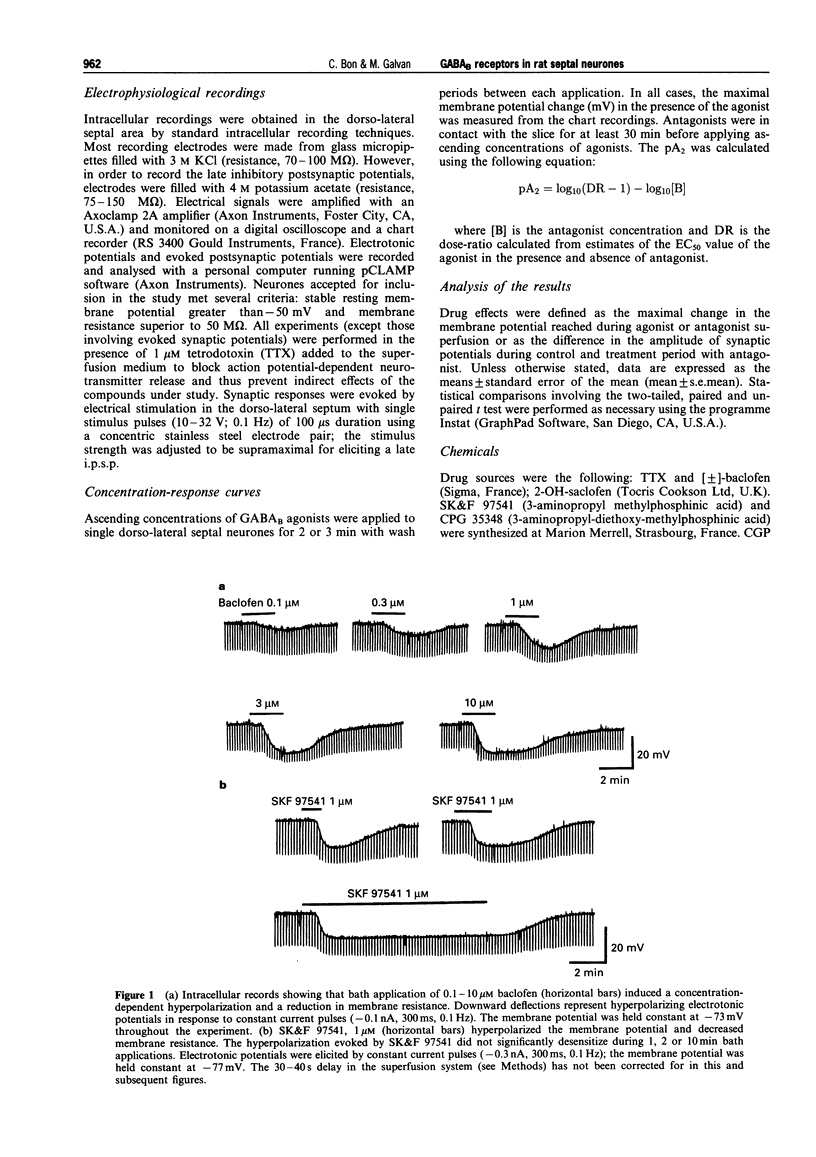
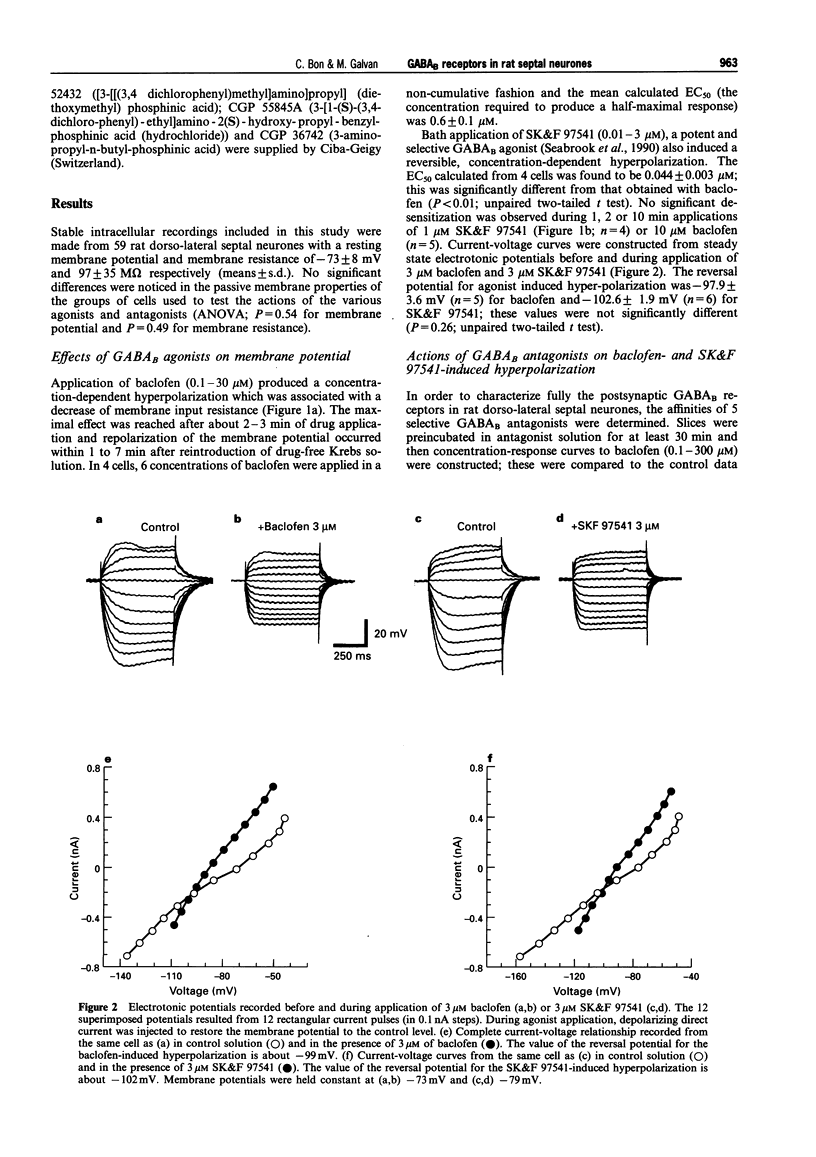
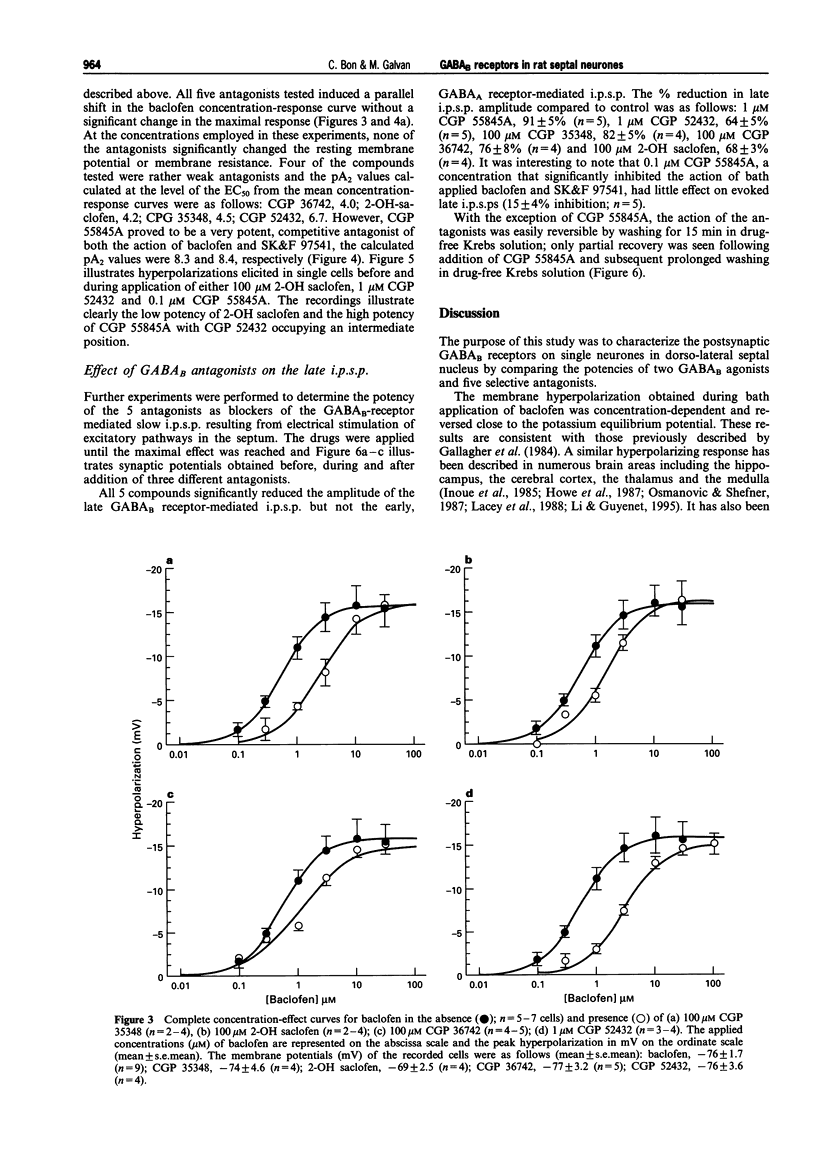
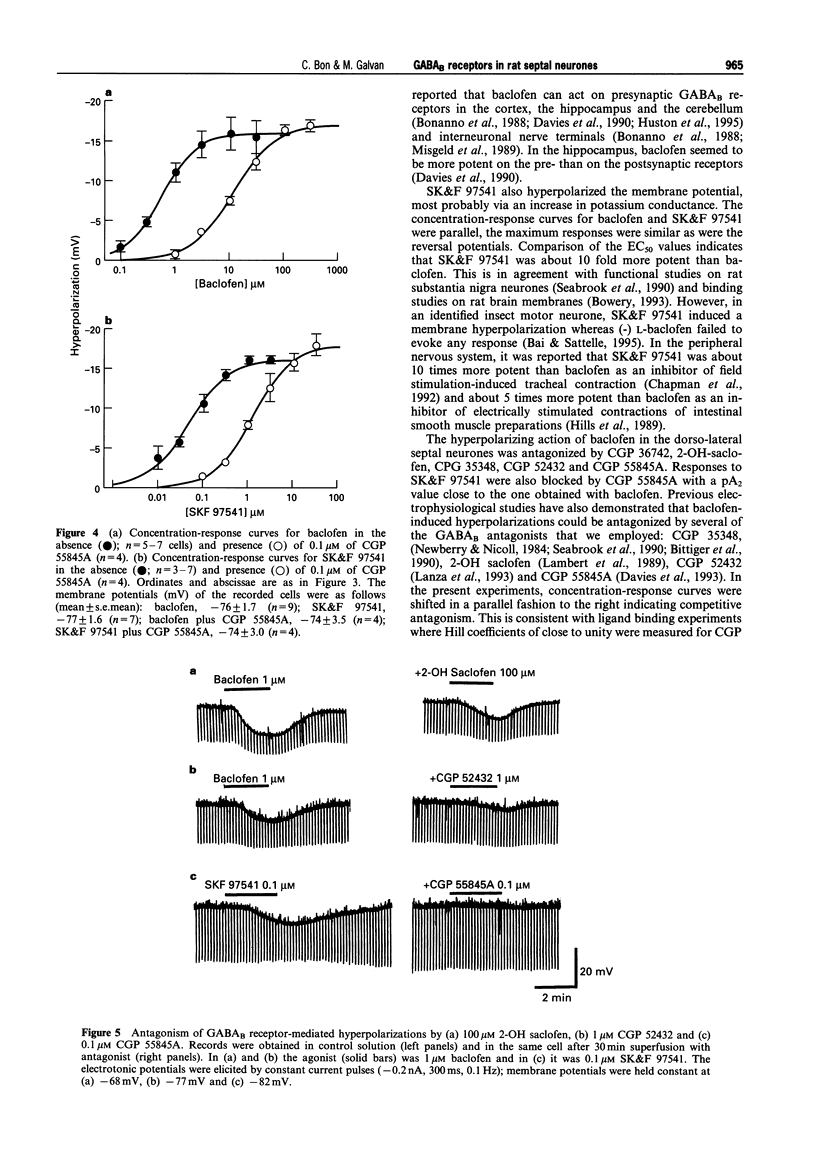
1. The actions of GABAB-receptor agonists and antagonists on rat dorso-lateral septal neurones in vitro were recorded with intracellular microelectrodes. 2. In the presence of 1 microM tetrodotoxin to prevent indirect neuronal effects caused by action potential-dependent neurotransmitter release, bath application of baclofen (0.1-30 microM) or SK&F 97541 (0.01-3 microM) evoked concentration-dependent hyperpolarizations which reversed close to the potassium equilibrium potential; the EC50S were 0.55 and 0.05 microM, respectively. No significant desensitization was observed during prolonged agonist exposure (< or = 10 min). 3. Hyperpolarizations induced by baclofen were antagonized in a competitive manner by the following GABAB-receptors antagonists (calculated pA2 values in parentheses): CGP 36742 (4.0), 2-OH saclofen (4.2), CGP 35348 (4.5), CGP 52432 (6.7) and CGP 55845A (8.3). Responses to SK&F 97541 were also antagonized by CGP 55845A (pA2 = 8.4). 4. The amplitude of the late, GABAB receptor-mediated inhibitory postsynaptic potential (i.p.s.p.) was reduced by the GABAB antagonists as follows (means +/- s.e.mean): CGP 55845A (1 microM) 91 +/- 5%, CGP 52432 (1 microM) 64 +/- 5%, CGP 35348 (100 microM) 82 +/- 5%, CGP 36742 (100 microM) 76 +/- 8%, and 2-OH saclofen (100 microM) 68 +/- 3%. 5. It is concluded that neurones in the rat dorso-lateral septal nucleus express conventional GABAB receptors, which are involved in the generation of slow inhibitory postsynaptic potentials. CGP 55845A is the most potent GABAB receptor antagonist described in this brain area.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai D, Sattelle D. A GABAB receptor on an identified insect motor neurone. J Exp Biol. 1995;198(Pt 4):889–894. doi: 10.1242/jeb.198.4.889. [DOI] [PubMed] [Google Scholar]
- Beck S. G., Birnstiel S., Pouliot W. A., Choi K. C. Baclofen concentration-response curves differ between hippocampal subfields. Neuroreport. 1995 Jan 26;6(2):310–312. doi: 10.1097/00001756-199501000-00021. [DOI] [PubMed] [Google Scholar]
- Bolser D. C., Blythin D. J., Chapman R. W., Egan R. W., Hey J. A., Rizzo C., Kuo S. C., Kreutner W. The pharmacology of SCH 50911: a novel, orally-active GABA-beta receptor antagonist. J Pharmacol Exp Ther. 1995 Sep;274(3):1393–1398. [PubMed] [Google Scholar]
- Bonanno G., Fontana G., Raiteri M. Phaclofen antagonizes GABA at autoreceptors regulating release in rat cerebral cortex. Eur J Pharmacol. 1988 Sep 13;154(2):223–224. doi: 10.1016/0014-2999(88)90104-5. [DOI] [PubMed] [Google Scholar]
- Bonanno G., Raiteri M. Functional evidence for multiple gamma-aminobutyric acidB receptor subtypes in the rat cerebral cortex. J Pharmacol Exp Ther. 1992 Jul;262(1):114–118. [PubMed] [Google Scholar]
- Bowery N. G. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987 Feb;20(2):365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Colmers W. F., Williams J. T. Pertussis toxin pretreatment discriminates between pre- and postsynaptic actions of baclofen in rat dorsal raphe nucleus in vitro. Neurosci Lett. 1988 Nov 11;93(2-3):300–306. doi: 10.1016/0304-3940(88)90099-7. [DOI] [PubMed] [Google Scholar]
- Costa E., Panula P., Thompson H. K., Cheney D. L. The transsynaptic regulation of the septal-hippocampal cholinergic neurons. Life Sci. 1983 Jan 17;32(3):165–179. doi: 10.1016/0024-3205(83)90028-0. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. H., Pozza M. F., Collingridge G. L. CGP 55845A: a potent antagonist of GABAB receptors in the CA1 region of rat hippocampus. Neuropharmacology. 1993 Oct;32(10):1071–1073. doi: 10.1016/0028-3908(93)90073-c. [DOI] [PubMed] [Google Scholar]
- DeFrance J. F., Yoshihara H., McCrea R. A., Kitai S. T. Pharmacology of the inhibiton in the lateral septal region. Exp Neurol. 1975 Sep;48(3 Pt 1):502–523. doi: 10.1016/0014-4886(75)90009-6. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Calcium channel currents and their inhibition by (-)-baclofen in rat sensory neurones: modulation by guanine nucleotides. J Physiol. 1987 May;386:1–17. doi: 10.1113/jphysiol.1987.sp016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froestl W., Mickel S. J., von Sprecher G., Diel P. J., Hall R. G., Maier L., Strub D., Melillo V., Baumann P. A., Bernasconi R. Phosphinic acid analogues of GABA. 2. Selective, orally active GABAB antagonists. J Med Chem. 1995 Aug 18;38(17):3313–3331. doi: 10.1021/jm00017a016. [DOI] [PubMed] [Google Scholar]
- Guyon A., Leresche N. Modulation by different GABAB receptor types of voltage-activated calcium currents in rat thalamocortical neurones. J Physiol. 1995 May 15;485(Pt 1):29–42. doi: 10.1113/jphysiol.1995.sp020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N. L., Lovinger D. M., Lambert N. A., Teyler T. J., Prager R., Ong J., Kerr D. I. The actions of 2-hydroxy-saclofen at presynaptic GABAB receptors in the rat hippocampus. Neurosci Lett. 1990 Nov 13;119(2):272–276. doi: 10.1016/0304-3940(90)90851-y. [DOI] [PubMed] [Google Scholar]
- Hasuo H., Gallagher J. P. Comparison of antagonism by phaclofen of baclofen induced hyperpolarizations and synaptically mediated late hyperpolarizing potentials recorded intracellularly from rat dorsolateral septal neurons. Neurosci Lett. 1988 Mar 21;86(1):77–81. doi: 10.1016/0304-3940(88)90186-3. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G., Hudson A. L. Inhibition of GABAB receptor binding by guanyl nucleotides. J Neurochem. 1984 Mar;42(3):652–657. doi: 10.1111/j.1471-4159.1984.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Hills J. M., Dingsdale R. A., Parsons M. E., Dolle R. E., Howson W. 3-Aminopropylphosphinic acid--a potent, selective GABAB receptor agonist in the guinea-pig ileum and rat anococcygeus muscle. Br J Pharmacol. 1989 Aug;97(4):1292–1296. doi: 10.1111/j.1476-5381.1989.tb12591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston E., Cullen G. P., Burley J. R., Dolphin A. C. The involvement of multiple calcium channel sub-types in glutamate release from cerebellar granule cells and its modulation by GABAB receptor activation. Neuroscience. 1995 Sep;68(2):465–478. doi: 10.1016/0306-4522(95)00172-f. [DOI] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Possible involvement of K+-conductance in the action of gamma-aminobutyric acid in the guinea-pig hippocampus. Br J Pharmacol. 1985 Oct;86(2):515–524. doi: 10.1111/j.1476-5381.1985.tb08923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Prager R. H., Gynther B. D., Curtis D. R. Phaclofen: a peripheral and central baclofen antagonist. Brain Res. 1987 Mar 3;405(1):150–154. doi: 10.1016/0006-8993(87)90999-1. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988 Jul;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N. A., Harrison N. L., Kerr D. I., Ong J., Prager R. H., Teyler T. J. Blockade of the late IPSP in rat CA1 hippocampal neurons by 2-hydroxy-saclofen. Neurosci Lett. 1989 Dec 15;107(1-3):125–128. doi: 10.1016/0304-3940(89)90803-3. [DOI] [PubMed] [Google Scholar]
- Lanza M., Fassio A., Gemignani A., Bonanno G., Raiteri M. CGP 52432: a novel potent and selective GABAB autoreceptor antagonist in rat cerebral cortex. Eur J Pharmacol. 1993 Jun 24;237(2-3):191–195. doi: 10.1016/0014-2999(93)90268-m. [DOI] [PubMed] [Google Scholar]
- Li Y. W., Guyenet P. G. Neuronal inhibition by a GABAB receptor agonist in the rostral ventrolateral medulla of the rat. Am J Physiol. 1995 Feb;268(2 Pt 2):R428–R437. doi: 10.1152/ajpregu.1995.268.2.R428. [DOI] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. Gamma-aminobutyric acid and inhibition in the septal nuclei of the rat. J Physiol. 1974 Mar;237(3):625–633. doi: 10.1113/jphysiol.1974.sp010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld U., Müller W., Brunner H. Effects of (-)baclofen on inhibitory neurons in the guinea pig hippocampal slice. Pflugers Arch. 1989 Jun;414(2):139–144. doi: 10.1007/BF00580955. [DOI] [PubMed] [Google Scholar]
- Möhler H. GABAergic synaptic transmission. Regulation by drugs. Arzneimittelforschung. 1992 Feb;42(2A):211–214. [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Karlsson G., Pozza M. F., Brugger F., Steinmann M., Van Riezen H., Fagg G., Hall R. G., Froestl W., Bittiger H. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990 Oct 2;187(1):27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Steinmann M. W., Ferrat T., Pozza M. F., Greiner K., Brugger F., Froestl W., Mickel S. J., Bittiger H. The actions of orally active GABAB receptor antagonists on GABAergic transmission in vivo and in vitro. Eur J Pharmacol. 1993 Mar 23;233(2-3):179–186. doi: 10.1016/0014-2999(93)90048-m. [DOI] [PubMed] [Google Scholar]
- Osmanović S. S., Shefner S. A. Baclofen increases the potassium conductance of rat locus coeruleus neurons recorded in brain slices. Brain Res. 1988 Jan 12;438(1-2):124–136. doi: 10.1016/0006-8993(88)91331-5. [DOI] [PubMed] [Google Scholar]
- Panula P., Revuelta A. V., Cheney D. L., Wu J. Y., Costa E. An immunohistochemical study on the location of GABAergic neurons in rat septum. J Comp Neurol. 1984 Jan 1;222(1):69–80. doi: 10.1002/cne.902220107. [DOI] [PubMed] [Google Scholar]
- Potashner S. J. Baclofen: effects on amino acid release and metabolism in slices of guinea pig cerebral cortex. J Neurochem. 1979 Jan;32(1):103–109. doi: 10.1111/j.1471-4159.1979.tb04516.x. [DOI] [PubMed] [Google Scholar]
- Raisman G. The connexions of the septum. Brain. 1966 Jun;89(2):317–348. doi: 10.1093/brain/89.2.317. [DOI] [PubMed] [Google Scholar]
- Scherer R. W., Ferkany J. W., Enna S. J. Evidence for pharmacologically distinct subsets of GABAB receptors. Brain Res Bull. 1988 Sep;21(3):439–443. doi: 10.1016/0361-9230(88)90156-6. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Howson W., Lacey M. G. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990 Dec;101(4):949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. In vitro studies of the role of gamma-aminobutyric acid in inhibition in the lateral septum of the rat. Synapse. 1987;1(2):184–190. doi: 10.1002/syn.890010206. [DOI] [PubMed] [Google Scholar]
- Waldmeier P. C., Wicki P., Feldtrauer J. J., Mickel S. J., Bittiger H., Baumann P. A. GABA and glutamate release affected by GABAB receptor antagonists with similar potency: no evidence for pharmacologically different presynaptic receptors. Br J Pharmacol. 1994 Dec;113(4):1515–1521. doi: 10.1111/j.1476-5381.1994.tb17168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


