Abstract
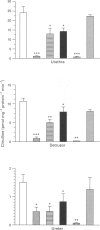
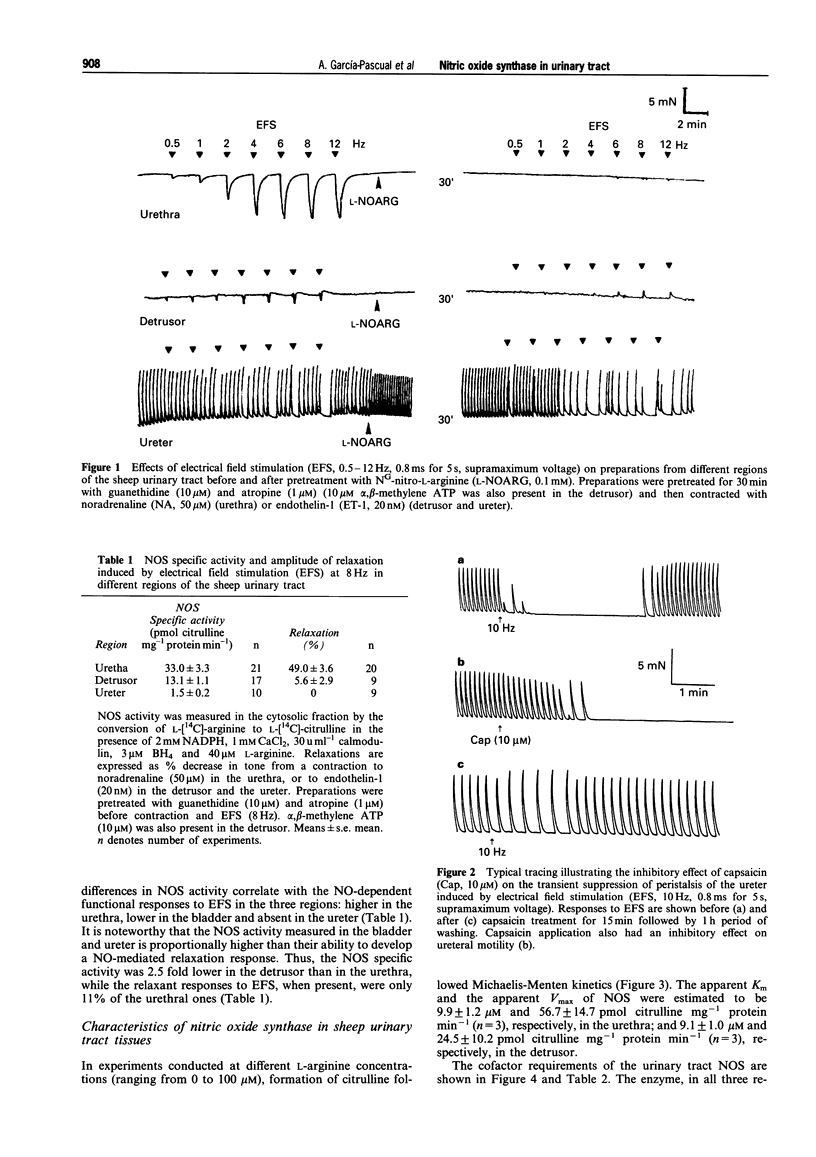
1. To define further the role of nitric oxide (NO) in urinary tract function, we have measured the presence of nitric oxide synthase (NOS) activity, and its relationship with functional NO-mediated responses to electrical field stimulation (EFS) in the urethra, the detrusor and the ureter from sheep. NOS activity was assayed by the conversion of L-[14C]-arginine to L-[14C]-citrulline. Endogenous production of citrulline was confirmed by thin layer chromatography. 2. NOS enzymatic activity was detected in the cytosolic fraction from tissue homogenates with the following regional distribution (pmol citrulline mg-1 protein min-1): urethra (33 +/- 3.3), detrusor (13.1 +/- 1.1) and ureter (1.5 +/- 0.2). No activity was detected in the particulate fraction of any region. 3. NOS activity was dependent on Ca(2+)-calmodulin and required exogenously added NADPH and tetrahydrobyoptein (BH4) for maximal activity. Exclusion of calmodulin from the incubation mixture did not modify NOS activity, but it was significantly reduced in the presence of the calmodulin antagonist, calmidazolium, suggesting the presence of enough endogenous calmodulin to sustain the observed NOS activity. 4. NOS activity was inhibited to a greater extent by NG-nitro-L-arginine (L-NOARG) and its methyl ester (L-NAME) than by NG-monomethyl-L-arginine (L-NMMA), while 7-nitroindazole (7-NI) was a weak inhibitor and L-cannavine had no effect. 5. Citrulline formation could be inhibited by superoxide dismutase in an oxyhaemoglobin-sensitive manner, suggesting feedback inhibition of NOS by NO. 6. EFS induced prominent NO-mediated relaxations in the urethra while minor or no responses were observed in the detrusor and the ureter, respectively. Urethral relaxations to EFS were inhibited by NOS inhibitors with the rank order of potency: L-NOARG = L-NAME > 7-NI > L-NMMA. 7. In conclusion, we have demonstrated the presence of NO-synthesizing enzymatic activity in the sheep urinary tract which shows similar characteristics to the constitutive NOS isoform found in brain. We suggest that the enzymatic activity measured in the urethral muscle layer may account for the NO-mediated urethral relaxation during micturition whereas regulation of detrusor and ureteral motor function by NOS containing nerves is less likely.
Full text
PDF


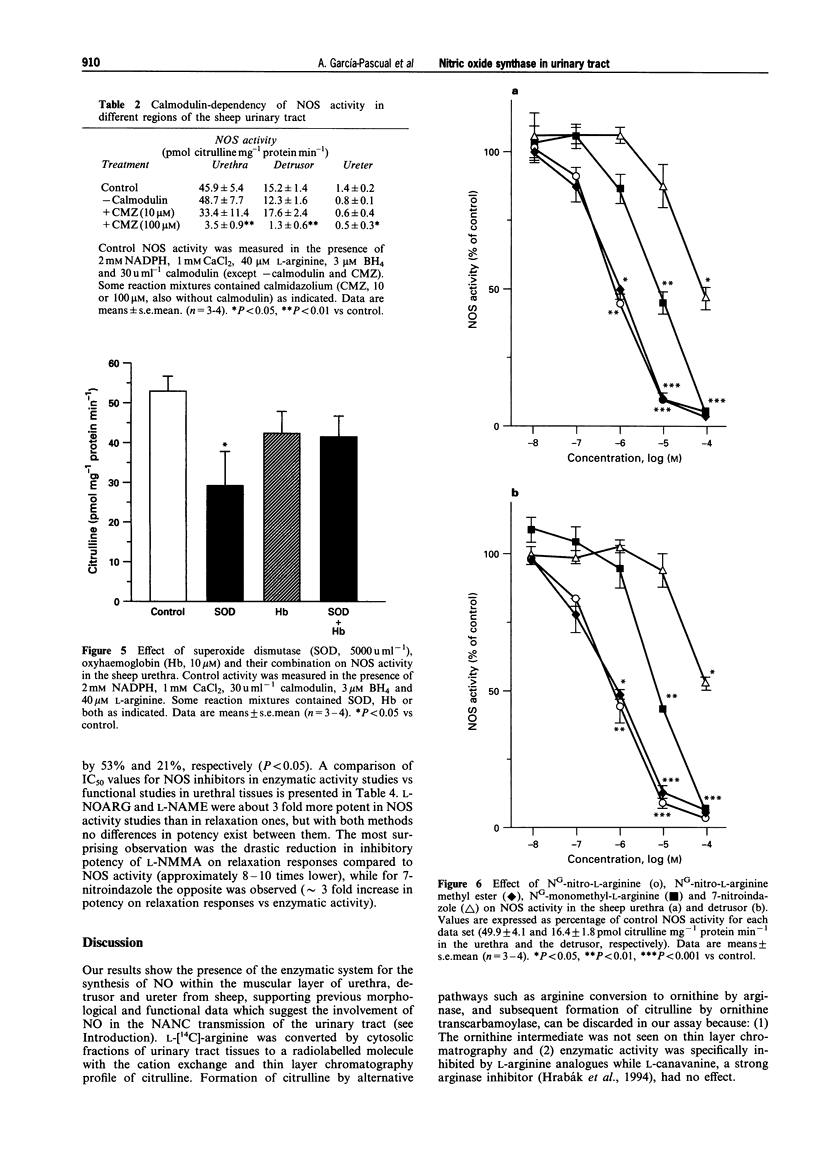
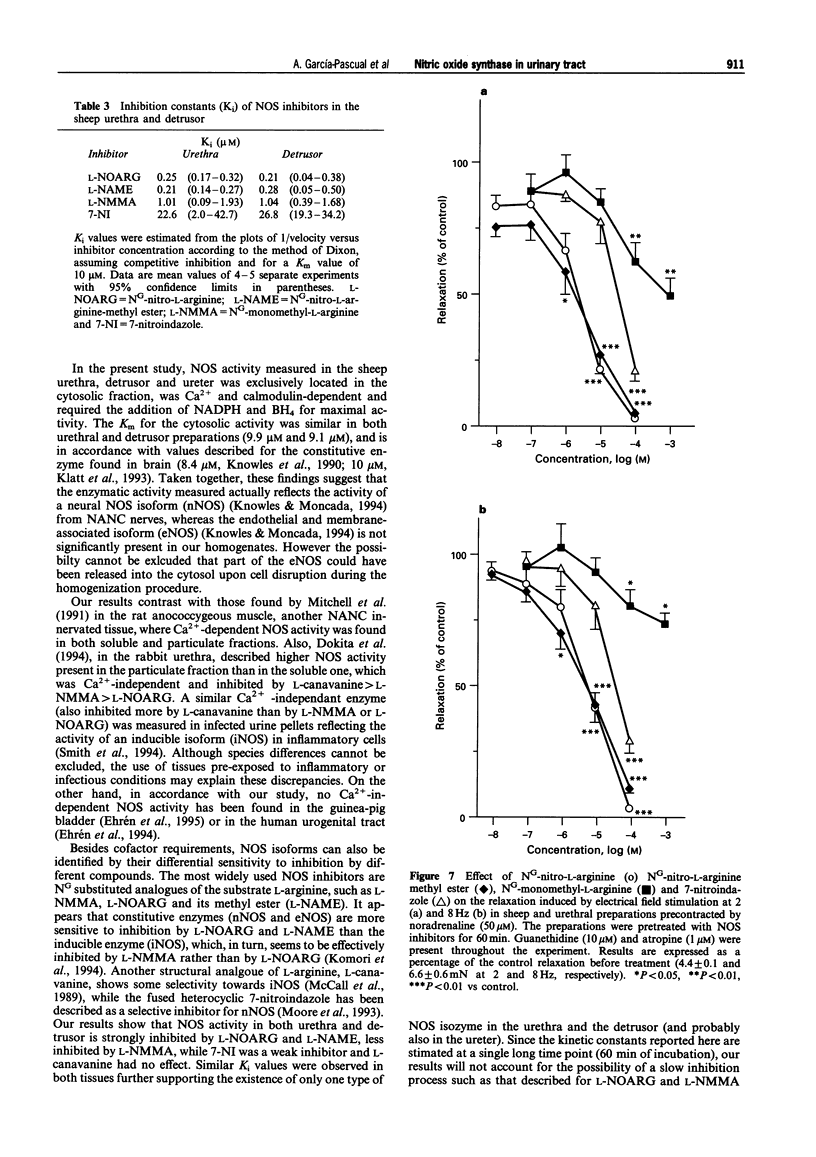



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allawi H. S., Wallace P., Pitcher A., Gaffen Z., Bland-Ward P. A., Moore P. K. Effect of 7-nitro indazole on neurotransmission in the rat vas deferens: mechanisms unrelated to inhibition of nitric oxide synthase. Br J Pharmacol. 1994 Sep;113(1):282–288. doi: 10.1111/j.1476-5381.1994.tb16206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993 Sep;45(3):253–308. [PubMed] [Google Scholar]
- Andersson K. E., Garcia Pascual A., Forman A., Tøttrup A. Non-adrenergic, non-cholinergic nerve-mediated relaxation of rabbit urethra is caused by nitric oxide. Acta Physiol Scand. 1991 Jan;141(1):133–134. doi: 10.1111/j.1748-1716.1991.tb09056.x. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Garcia Pascual A., Persson K., Forman A., Tøttrup A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J Urol. 1992 Jan;147(1):253–259. doi: 10.1016/s0022-5347(17)37208-7. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokita S., Smith S. D., Nishimoto T., Wheeler M. A., Weiss R. M. Involvement of nitric oxide and cyclic GMP in rabbit urethral relaxation. Eur J Pharmacol. 1994 Feb 15;266(3):269–275. doi: 10.1016/0922-4106(94)90136-8. [DOI] [PubMed] [Google Scholar]
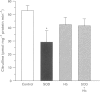
- Ehrén I., Adolfsson J., Wiklund N. P. Nitric oxide synthase activity in the human urogenital tract. Urol Res. 1994;22(5):287–290. doi: 10.1007/BF00297196. [DOI] [PubMed] [Google Scholar]
- Ehrén I., Hammarström M., Adolfsson J., Wiklund N. P. Induction of calcium-dependent nitric oxide synthase by sex hormones in the guinea-pig urinary bladder. Acta Physiol Scand. 1995 Apr;153(4):393–394. doi: 10.1111/j.1748-1716.1995.tb09877.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Pascual A., Costa G., Garcia-Sacristan A., Andersson K. E. Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol Scand. 1991 Apr;141(4):531–539. doi: 10.1111/j.1748-1716.1991.tb09114.x. [DOI] [PubMed] [Google Scholar]
- García-Pascual A., Triguero D. Relaxation mechanisms induced by stimulation of nerves and by nitric oxide in sheep urethral muscle. J Physiol. 1994 Apr 15;476(2):333–347. doi: 10.1113/jphysiol.1994.sp020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessl C., Grozdanovic Z., Knispel H. H., Wegner H. E., Miller K. Nitroxergic innervation of the human ureterovesical junction. Urol Res. 1995;23(3):189–192. doi: 10.1007/BF00389572. [DOI] [PubMed] [Google Scholar]
- Hernández M., Prieto D., Orensanz L. M., Barahona M. V., García-Sacristán A., Simonsen U. Nitric oxide is involved in the non-adrenergic, non-cholinergic inhibitory neurotransmission of the pig intravesical ureter. Neurosci Lett. 1995 Feb 15;186(1):33–36. doi: 10.1016/0304-3940(94)11275-n. [DOI] [PubMed] [Google Scholar]
- Hrabák A., Bajor T., Temesi A. Comparison of substrate and inhibitor specificity of arginase and nitric oxide (NO) synthase for arginine analogues and related compounds in murine and rat macrophages. Biochem Biophys Res Commun. 1994 Jan 14;198(1):206–212. doi: 10.1006/bbrc.1994.1029. [DOI] [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Brunner F., Mayer B. Inhibitors of brain nitric oxide synthase. Binding kinetics, metabolism, and enzyme inactivation. J Biol Chem. 1994 Jan 21;269(3):1674–1680. [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Uray G., Mayer B. Multiple catalytic functions of brain nitric oxide synthase. Biochemical characterization, cofactor-requirement, and the role of N omega-hydroxy-L-arginine as an intermediate. J Biol Chem. 1993 Jul 15;268(20):14781–14787. [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Kinetic characteristics of nitric oxide synthase from rat brain. Biochem J. 1990 Jul 1;269(1):207–210. doi: 10.1042/bj2690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori Y., Wallace G. C., Fukuto J. M. Inhibition of purified nitric oxide synthase from rat cerebellum and macrophage by L-arginine analogs. Arch Biochem Biophys. 1994 Dec;315(2):213–218. doi: 10.1006/abbi.1994.1492. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leone A. M., Wiklund N. P., Hökfelt T., Brundin L., Moncada S. Release of nitric oxide by nerve stimulation in the human urogenital tract. Neuroreport. 1994 Feb 24;5(6):733–736. doi: 10.1097/00001756-199402000-00019. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S. The neurotransmitter role of calcitonin gene-related peptide in the rat and guinea-pig ureter: effect of a calcitonin gene-related peptide antagonist and species-related differences in the action of omega conotoxin on calcitonin gene-related peptide release from primary afferents. Neuroscience. 1991;43(1):261–268. doi: 10.1016/0306-4522(91)90433-o. [DOI] [PubMed] [Google Scholar]
- Mayer B., Klatt P., Werner E. R., Schmidt K. Kinetics and mechanism of tetrahydrobiopterin-induced oxidation of nitric oxide. J Biol Chem. 1995 Jan 13;270(2):655–659. doi: 10.1074/jbc.270.2.655. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill D. L., Traugh N. E., Jr, Vaidya A. M., Hua H. T., Papka R. E. Origin and distribution of NADPH-diaphorase-positive neurons and fibers innervating the urinary bladder of the rat. Neurosci Lett. 1992 Nov 23;147(1):33–36. doi: 10.1016/0304-3940(92)90768-3. [DOI] [PubMed] [Google Scholar]
- Mevorach R. A., Bogaert G. A., Kogan B. A. Role of nitric oxide in fetal lower urinary tract function. J Urol. 1994 Aug;152(2 Pt 1):510–514. doi: 10.1016/s0022-5347(17)32784-2. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Sheng H., Förstermann U., Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., Wallace P., Gaffen Z., Hart S. L., Babbedge R. C. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects. Br J Pharmacol. 1993 Sep;110(1):219–224. doi: 10.1111/j.1476-5381.1993.tb13795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. E., Noack E. Nitric oxide assay using hemoglobin method. Methods Enzymol. 1994;233:240–250. doi: 10.1016/s0076-6879(94)33027-1. [DOI] [PubMed] [Google Scholar]
- Persson K., Alm P., Johansson K., Larsson B., Andersson K. E. Nitric oxide synthase in pig lower urinary tract: immunohistochemistry, NADPH diaphorase histochemistry and functional effects. Br J Pharmacol. 1993 Oct;110(2):521–530. doi: 10.1111/j.1476-5381.1993.tb13842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K., Andersson K. E. Nitric oxide and relaxation of pig lower urinary tract. Br J Pharmacol. 1992 Jun;106(2):416–422. doi: 10.1111/j.1476-5381.1992.tb14349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K., Igawa Y., Mattiasson A., Andersson K. E. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br J Pharmacol. 1992 Sep;107(1):178–184. doi: 10.1111/j.1476-5381.1992.tb14483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet P. J., Edyvane K. A., Jonavicius J., Marshall V. R. Colocalization of nitric oxide synthase with vasoactive intestinal peptide, neuropeptide Y, and tyrosine hydroxylase in nerves supplying the human ureter. J Urol. 1994 Oct;152(4):1292–1296. doi: 10.1016/s0022-5347(17)32570-3. [DOI] [PubMed] [Google Scholar]
- Smet P. J., Edyvane K. A., Jonavicius J., Marshall V. R. Distribution of NADPH-diaphorase-positive nerves supplying the human urinary bladder. J Auton Nerv Syst. 1994 Apr;47(1-2):109–113. doi: 10.1016/0165-1838(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Smith S. D., Wheeler M. A., Weiss R. M. Nitric oxide synthase: an endogenous source of elevated nitrite in infected urine. Kidney Int. 1994 Feb;45(2):586–591. doi: 10.1038/ki.1994.76. [DOI] [PubMed] [Google Scholar]
- Triguero D., Prieto D., García-Pascual A. NADPH-diaphorase and NANC relaxations are correlated in the sheep urinary tract. Neurosci Lett. 1993 Nov 26;163(1):93–96. doi: 10.1016/0304-3940(93)90237-f. [DOI] [PubMed] [Google Scholar]
- Vizzard M. A., Erdman S. L., Förstermann U., de Groat W. C. Differential distribution of nitric oxide synthase in neural pathways to the urogenital organs (urethra, penis, urinary bladder) of the rat. Brain Res. 1994 May 23;646(2):279–291. doi: 10.1016/0006-8993(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Lubeskie A., Umansky S. The inhibition of the constitutive bovine endothelial nitric oxide synthase by imidazole and indazole agents. Arch Biochem Biophys. 1994 Nov 1;314(2):360–366. doi: 10.1006/abbi.1994.1454. [DOI] [PubMed] [Google Scholar]