Abstract
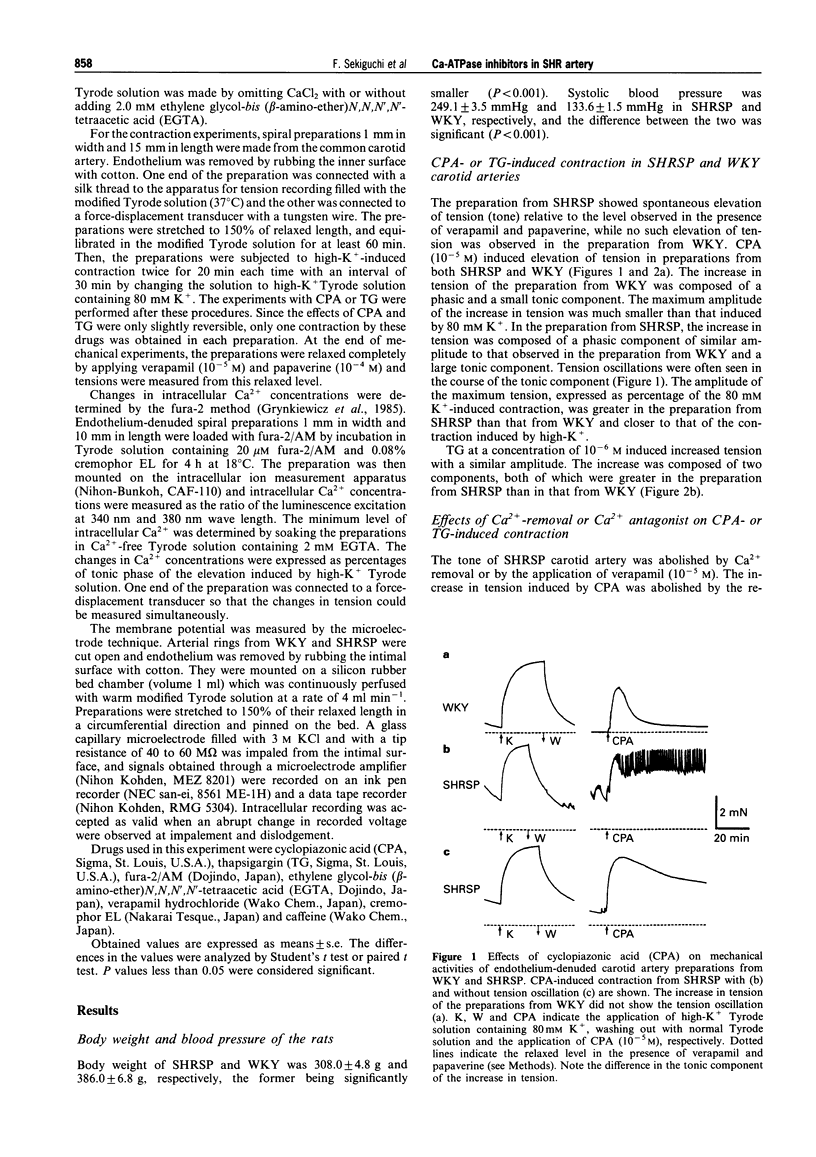
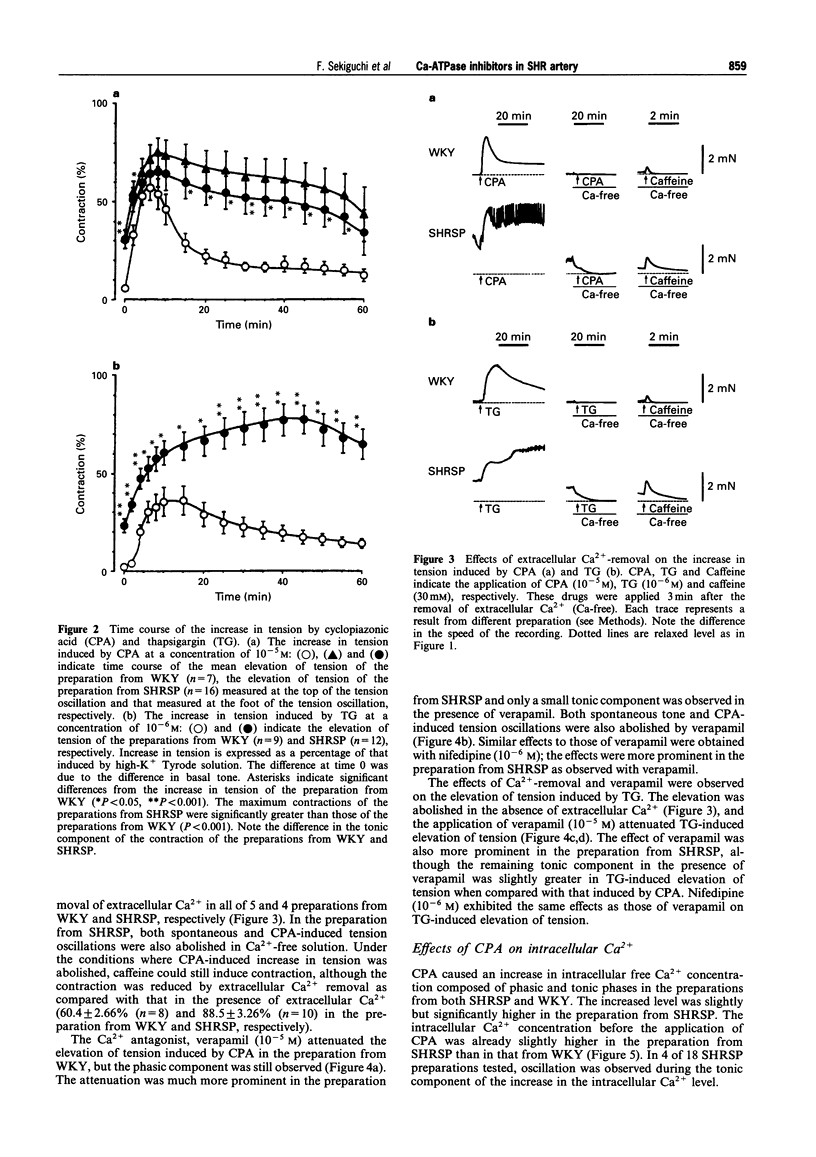
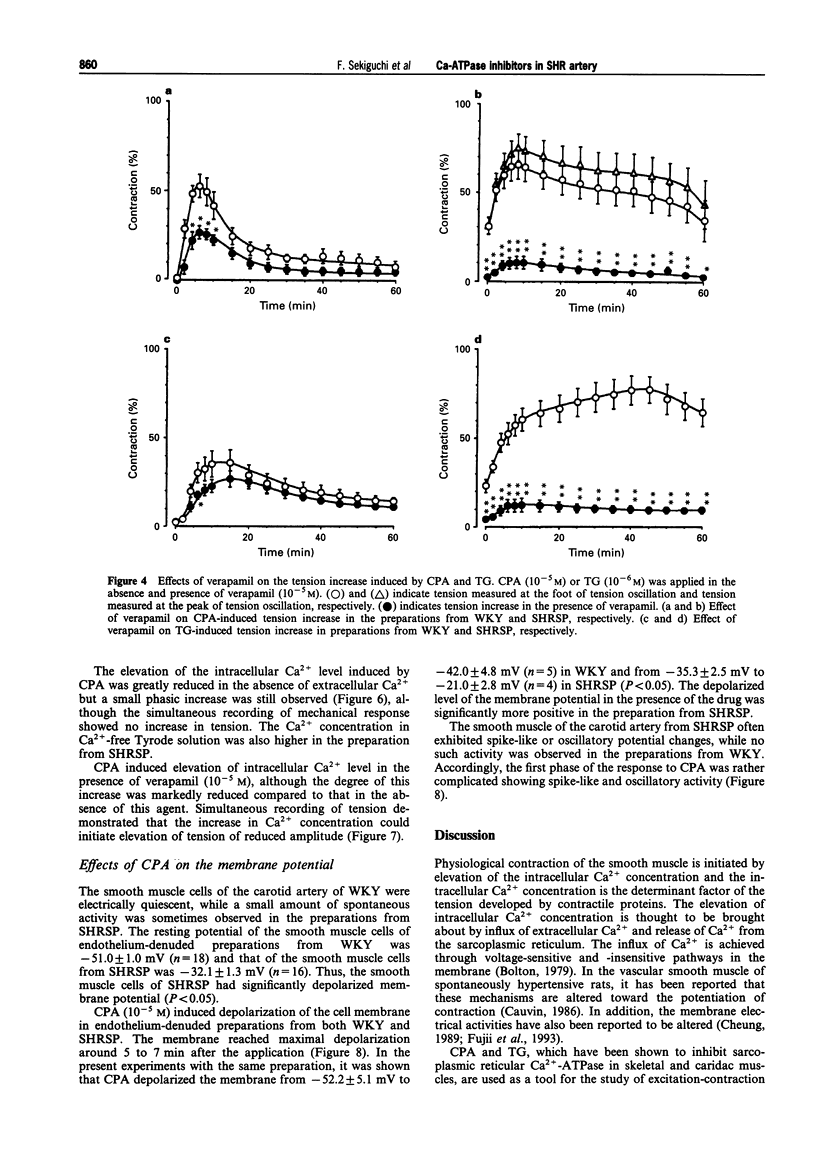
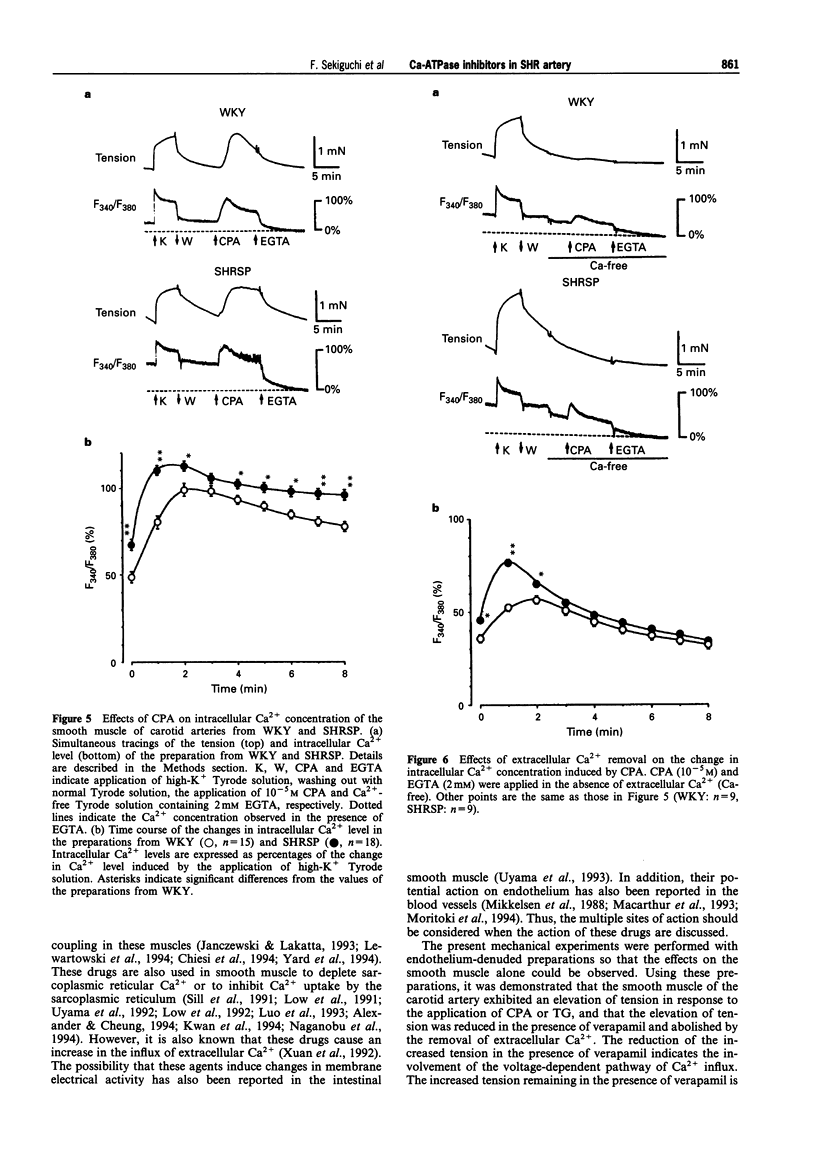
1. The effects of cyclopiazonic acid (CPA) and thapsigargin (TG), both of which are known to inhibit sarcoplasmic reticular Ca(2+)-ATPase, on the mechanical activities, intracellular Ca2+ level and electrical activities of smooth muscle of the carotid artery of stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar Kyoto rats (WKY) were compared. 2. Both CPA and TG induced elevation of tension of the smooth muscle, which was composed of a phasic and a tonic component. The level of tension attained, especially the tonic component, was greater in the preparation from SHRSP. 3. The elevation of tension was associated with an increased intracellular Ca2+ level. Both the elevation of tension and the increase in intracellular Ca2+ were diminished by the removal of extracellular Ca2+ or by the application of verapamil. 4. The resting membrane potential of the preparations from SHRSP were depolarized to a greater extent than those from WKY.CPA depolarized the smooth muscle from both SHRSP and WKY, and the final level was also more depolarized in the preparation from SHRSP. 5. These results indicate that the elevation of tension induced by these drugs is mainly due to increased Ca2+ influx through voltage-dependent Ca2+ channels, and the difference in the action between the preparation from SHRSP and that from WKY can be explained mainly by the changes in the channels. 6. Thus, differences in the action of these drugs on the tension of smooth muscle between preparations from WKY and SHRSP can mainly be explained by the difference in the membrane potential which is related to the difference in voltage-dependent Ca2+ influx.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P. B., Cheung D. W. Ca2+ mobilization by caffeine in single smooth muscle cells of the rat tail artery. Eur J Pharmacol. 1994 Dec 15;288(1):79–88. doi: 10.1016/0922-4106(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Baró I., Eisner D. A. The effects of thapsigargin on [Ca2+]i in isolated rat mesenteric artery vascular smooth muscle cells. Pflugers Arch. 1992 Jan;420(1):115–117. doi: 10.1007/BF00378652. [DOI] [PubMed] [Google Scholar]
- Bian K., Bukoski R. D. Myofilament calcium sensitivity of normotensive and hypertensive resistance arteries. Hypertension. 1995 Jan;25(1):110–116. doi: 10.1161/01.hyp.25.1.110. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Wrzosek A., Grueninger S. The role of the sarcoplasmic reticulum in various types of cardiomyocytes. Mol Cell Biochem. 1994 Jan 26;130(2):159–171. doi: 10.1007/BF01457397. [DOI] [PubMed] [Google Scholar]
- Fujii K., Ohmori S., Tominaga M., Abe I., Takata Y., Ohya Y., Kobayashi K., Fujishima M. Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am J Physiol. 1993 Aug;265(2 Pt 2):H509–H516. doi: 10.1152/ajpheart.1993.265.2.H509. [DOI] [PubMed] [Google Scholar]
- Gagov H. S., Duridanova D. B., Boev K. K. Participation of calcium, released from the IP3-sensitive Ca-store in activation of Ca-dependent potassium conductance of ileal smooth muscle cells. Gen Physiol Biophys. 1993 Jun;12(3):199–211. [PubMed] [Google Scholar]
- Gericke M., Droogmans G., Nilius B. Thapsigargin discharges intracellular calcium stores and induces transmembrane currents in human endothelial cells. Pflugers Arch. 1993 Mar;422(6):552–557. doi: 10.1007/BF00374001. [DOI] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989 Nov 15;38(22):3995–4003. doi: 10.1016/0006-2952(89)90679-5. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Janczewski A. M., Lakatta E. G. Thapsigargin inhibits Ca2+ uptake, and Ca2+ depletes sarcoplasmic reticulum in intact cardiac myocytes. Am J Physiol. 1993 Aug;265(2 Pt 2):H517–H522. doi: 10.1152/ajpheart.1993.265.2.H517. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y. Dysfunction of calcium handling by smooth muscle in hypertension. Can J Physiol Pharmacol. 1985 Apr;63(4):366–374. doi: 10.1139/y85-066. [DOI] [PubMed] [Google Scholar]
- Lewartowski B., Rózycka M., Janiak R. Effects of thapsigargin in normal and pretreated with ryanodine guinea pig cardiomyocytes. Am J Physiol. 1994 May;266(5 Pt 2):H1829–H1839. doi: 10.1152/ajpheart.1994.266.5.H1829. [DOI] [PubMed] [Google Scholar]
- Low A. M., Darby P. J., Kwan C. Y., Daniel E. E. Effects of thapsigargin and ryanodine on vascular contractility: cross-talk between sarcoplasmic reticulum and plasmalemma. Eur J Pharmacol. 1993 Jan 5;230(1):53–62. doi: 10.1016/0014-2999(93)90409-b. [DOI] [PubMed] [Google Scholar]
- Low A. M., Gaspar V., Kwan C. Y., Darby P. J., Bourreau J. P., Daniel E. E. Thapsigargin inhibits repletion of phenylephrine-sensitive intracellular Ca++ pool in vascular smooth muscles. J Pharmacol Exp Ther. 1991 Sep;258(3):1105–1113. [PubMed] [Google Scholar]
- Low A. M., Kwan C. Y., Daniel E. E. Evidence for two types of internal Ca2+ stores in canine mesenteric artery with different refilling mechanisms. Am J Physiol. 1992 Jan;262(1 Pt 2):H31–H37. doi: 10.1152/ajpheart.1992.262.1.H31. [DOI] [PubMed] [Google Scholar]
- Luo D. L., Nakazawa M., Ishibashi T., Kato K., Imai S. Putative, selective inhibitors of sarcoplasmic reticulum Ca+(+)-pump ATPase inhibit relaxation by nitroglycerin and atrial natriuretic factor of the rabbit aorta contracted by phenylephrine. J Pharmacol Exp Ther. 1993 Jun;265(3):1187–1192. [PubMed] [Google Scholar]
- Macarthur H., Hecker M., Busse R., Vane J. R. Selective inhibition of agonist-induced but not shear stress-dependent release of endothelial autacoids by thapsigargin. Br J Pharmacol. 1993 Jan;108(1):100–105. doi: 10.1111/j.1476-5381.1993.tb13446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Santicioli P. Effect of the Ca(2+)-ATPase inhibitor, cyclopiazonic acid, on electromechanical coupling in the guinea-pig ureter. Br J Pharmacol. 1995 Jan;114(1):127–137. doi: 10.1111/j.1476-5381.1995.tb14916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- Mikkelsen E. O., Thastrup O., Christensen S. B. Effects of thapsigargin in isolated rat thoracic aorta. Pharmacol Toxicol. 1988 Jan;62(1):7–11. doi: 10.1111/j.1600-0773.1988.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone and cyclopiazonic acid decrease the Ca2+ permeability of endoplasmic reticulum. Eur J Pharmacol. 1992 Dec 1;227(4):391–394. doi: 10.1016/0922-4106(92)90156-p. [DOI] [PubMed] [Google Scholar]
- Moritoki H., Hisayama T., Takeuchi S., Kondoh W., Imagawa M. Relaxation of rat thoracic aorta induced by the Ca(2+)-ATPase inhibitor, cyclopiazonic acid, possibly through nitric oxide formation. Br J Pharmacol. 1994 Mar;111(3):655–662. doi: 10.1111/j.1476-5381.1994.tb14788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganobu K., Takagi M., Kawasaki H., Ito K. Modification by cyclopiazonic acid and ryanodine of depolarization-induced constriction in rat mesenteric artery. Eur J Pharmacol. 1994 Jan 14;251(2-3):307–310. doi: 10.1016/0014-2999(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Neusser M., Golinski P., Zhu Z., Tepel M., Zidek W. Effects of protein kinase C activation on intracellular Ca2+ distribution in vascular smooth muscle cells of spontaneously hypertensive rats. J Vasc Res. 1993 Mar-Apr;30(2):116–120. doi: 10.1159/000158983. [DOI] [PubMed] [Google Scholar]
- Sasaki F., Osugi S., Shimamura K., Sunano S. Relationship between blood pressure and smooth muscle tone in aortae of hypertensive rats: roles of [Ca2+]. J Smooth Muscle Res. 1993 Jun;29(3):69–79. doi: 10.1540/jsmr.29.69. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Sill J. C., Uhl C., Eskuri S., Van Dyke R., Tarara J. Halothane inhibits agonist-induced inositol phosphate and Ca2+ signaling in A7r5 cultured vascular smooth muscle cells. Mol Pharmacol. 1991 Dec;40(6):1006–1013. [PubMed] [Google Scholar]
- Suzuki M., Muraki K., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca(2+)-pump, reduces Ca(2+)-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992 Sep;107(1):134–140. doi: 10.1111/j.1476-5381.1992.tb14475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Tepel M., Ruess C., Mehring N., Neusser M., Zidek W. Effect of inhibition of sarcoplasmic Ca(2+)-ATPase on vasoconstriction and cytosolic Ca2+ in aortic smooth muscle from spontaneously hypertensive and normotensive rats. Clin Exp Hypertens. 1994 Jul;16(4):493–506. doi: 10.3109/10641969409067958. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of Ca(2+)-ATPase in sarcoplasmic reticulum, increases excitability in ileal smooth muscle. Br J Pharmacol. 1993 Oct;110(2):565–572. doi: 10.1111/j.1476-5381.1993.tb13848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992 May;106(1):208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Y. T., Wang O. L., Whorton A. R. Thapsigargin stimulates Ca2+ entry in vascular smooth muscle cells: nicardipine-sensitive and -insensitive pathways. Am J Physiol. 1992 May;262(5 Pt 1):C1258–C1265. doi: 10.1152/ajpcell.1992.262.5.C1258. [DOI] [PubMed] [Google Scholar]
- Yard N. J., Chiesi M., Ball H. A. Effect of cyclopiazonic acid, an inhibitor of sarcoplasmic reticulum Ca(2+)-ATPase, on the frequency-dependence of the contraction-relaxation cycle of the guinea-pig isolated atrium. Br J Pharmacol. 1994 Nov;113(3):1001–1007. doi: 10.1111/j.1476-5381.1994.tb17092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Inazu M., Weir B., Buchanan M., Daniel E. Cyclopiazonic acid stimulates Ca2+ influx through non-specific cation channels in endothelial cells. Eur J Pharmacol. 1994 Jan 14;251(2-3):119–125. doi: 10.1016/0014-2999(94)90391-3. [DOI] [PubMed] [Google Scholar]


