Abstract
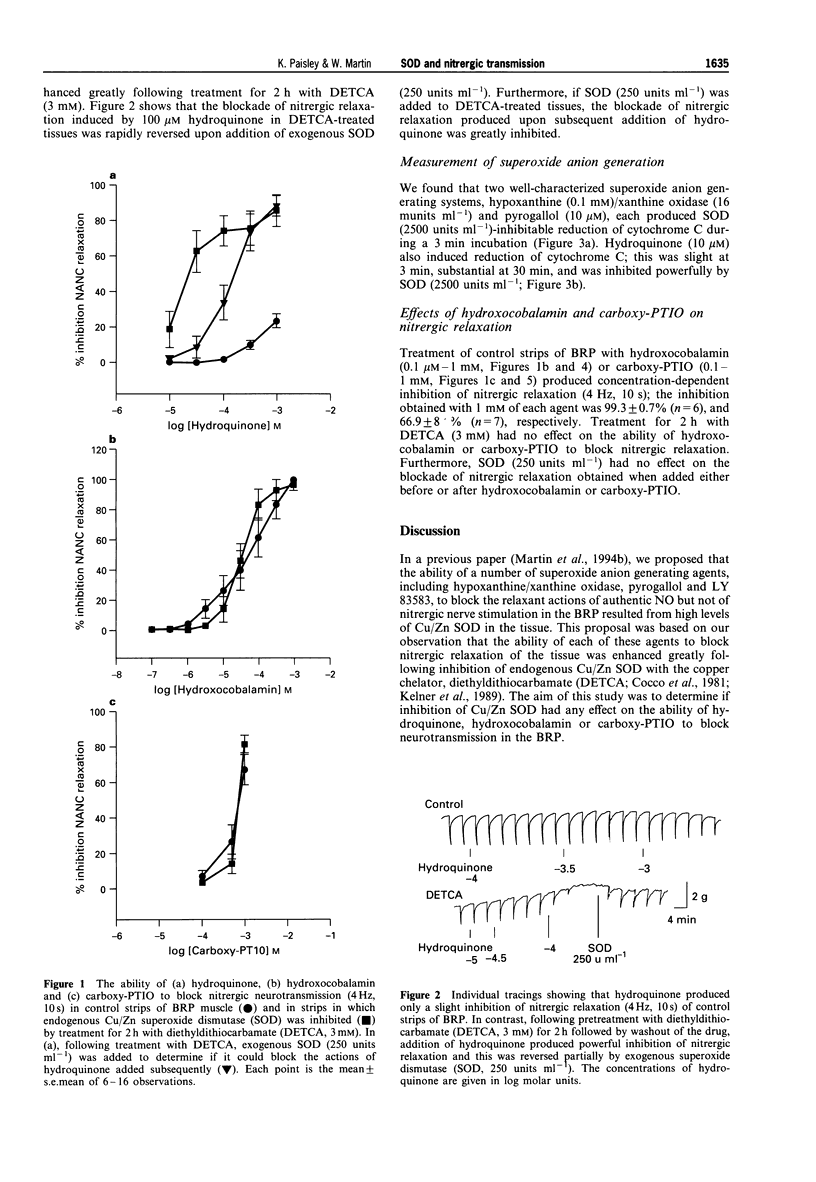
1. The effects of inhibiting endogenous Cu/Zn superoxide dismutase (SOD) with diethyldithiocarbamate (DETCA) were examined on the ability of hydroquinone, hydroxocobalamin and carboxy-PTIO to block nitrergic relaxation in the bovine retractor penis (BRP) muscle. 2. Incubation of strips of BRP with DETCA (3 mM) for 2 h reduced SOD activity from 73.1 +/- 15.7 to 8.2 +/- 1.9 units mg-1 protein. 3. Hydroquinone (10 microM--1 mM) produced weak inhibition of nitrergic (4 Hz, 10 s) relaxation in control strips of BRP, but powerful inhibition in strips treated with DETCA (3 mM, 2 h). Exogenous SOD (250 units ml--1) produced a partial blockade of the ability of hydroquinone to inhibit nitrergic relaxation in DETCA-treated strips. 4. In an assay of SOD-inhibitable reduction of cytochrome C, hypoxanthine (0.1 mM)/xanthine oxidase (16 munits ml-1) and pyrogallol (10 microM), led to the rapid generation of superoxide anion. Hydroquinone (10 microM) also led to the generation of the free radical, although the rate of generation was slower. 5. Two NO-scavenging agents, hydroxocobalamin (0.1 microM--1 mM) and carboxy-PTIO (0.1-1 mM), produced concentration-dependent blockade of nitrergic relaxation of the BRP. The magnitude of the blockade induced by these agents was unaffected following treatment with DETCA or SOD. 6. The findings with hydroquinone support our previous proposal that endogenous Cu/Zn SOD plays a vital role in protecting nitrergic neurotransmission from inactivation by superoxide anion. Results with hydroxocobalamin and carboxy-PTIO are consistent with the known ability of these agents to scavenge NO. The nitrergic neurotransmitter in the BRP thus appears to have the properties of NO.
Full text
PDF

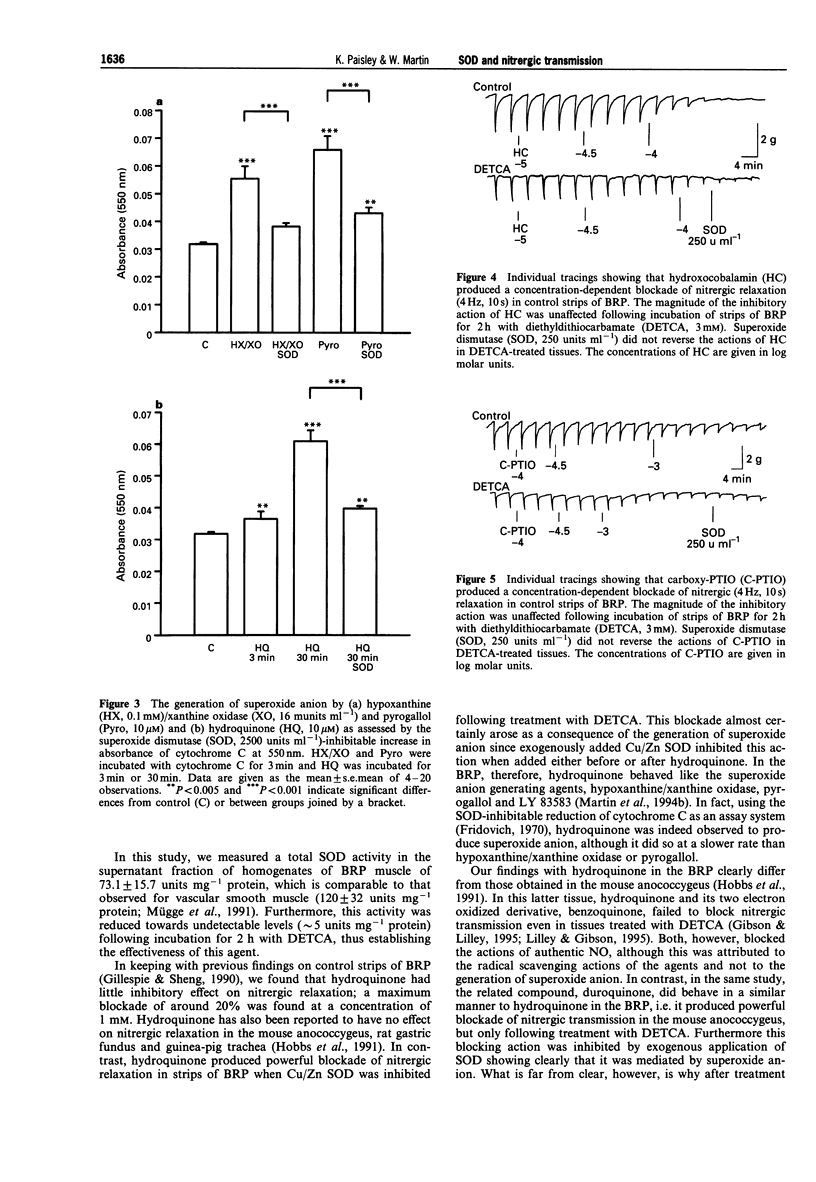


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Yoshida M., Miyamoto Y., Sato K., Kohno M., Sasamoto K., Miyazaki K., Ueda S., Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993 Jan 26;32(3):827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- Barbier A. J., Lefebvre R. A. Effect of LY 83583 on relaxation induced by non-adrenergic non-cholinergic nerve stimulation and exogenous nitric oxide in the rat gastric fundus. Eur J Pharmacol. 1992 Aug 25;219(2):331–334. doi: 10.1016/0014-2999(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Barbier A. J., Lefebvre R. A. Influence of S-nitrosothiols and nitrate tolerance in the rat gastric fundus. Br J Pharmacol. 1994 Apr;111(4):1280–1286. doi: 10.1111/j.1476-5381.1994.tb14884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma M. G., Balvers W. G., Boeren S., Vervoort J., Rietjens I. M. NADPH-cytochrome reductase catalysed redox cycling of 1,4-benzoquinone; hampered at physiological conditions, initiated at increased pH values. Biochem Pharmacol. 1994 Jun 1;47(11):1949–1955. doi: 10.1016/0006-2952(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Block of some non-adrenergic inhibitory responses of smooth muscle by a substance from haemolysed erythrocytes. J Physiol. 1982 Jul;328:11–25. doi: 10.1113/jphysiol.1982.sp014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Pollock D. Oxyhaemoglobin blocks non-adrenergic non-cholinergic inhibition in the bovine retractor penis muscle. Eur J Pharmacol. 1982 Nov 19;85(2):221–224. doi: 10.1016/0014-2999(82)90470-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cocco D., Calabrese L., Rigo A., Argese E., Rotilio G. Re-examination of the reaction of diethyldithiocarbamate with the copper of superoxide dismutase. J Biol Chem. 1981 Sep 10;256(17):8983–8986. [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscles. Br J Pharmacol. 1990 Jan;99(1):194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Tucker J. F., Gibson A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br J Pharmacol. 1991 Nov;104(3):645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner M. J., Bagnell R., Hale B., Alexander N. M. Inactivation of intracellular copper-zinc superoxide dismutase by copper chelating agents without glutathione depletion and methemoglobin formation. Free Radic Biol Med. 1989;6(4):355–360. doi: 10.1016/0891-5849(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Lilley E., Gibson A. Inhibition of relaxations to nitrergic stimulation of the mouse anococcygeus by duroquinone. Br J Pharmacol. 1995 Dec;116(8):3231–3236. doi: 10.1111/j.1476-5381.1995.tb15129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. R., Gillespie J. S., Gibson I. F., Martin W. Effects of NG-substituted analogues of L-arginine on NANC relaxation of the rat anococcygeus and bovine retractor penis muscles and the bovine penile artery. Br J Pharmacol. 1991 Sep;104(1):53–58. doi: 10.1111/j.1476-5381.1991.tb12384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Gillespie J. S., Martin W. Non-adrenergic, non-cholinergic relaxation of the bovine retractor penis muscle: role of S-nitrosothiols. Br J Pharmacol. 1994 Apr;111(4):1287–1295. doi: 10.1111/j.1476-5381.1994.tb14885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Martin W., McAllister K. H., Paisley K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994 Nov;33(11):1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Gryglewski R. J. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Rajanayagam M. A., Li C. G., Rand M. J. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br J Pharmacol. 1993 Jan;108(1):3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF. Br J Pharmacol. 1995 Sep;116(2):1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Wood J., Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994 Nov;33(11):1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]


