Abstract
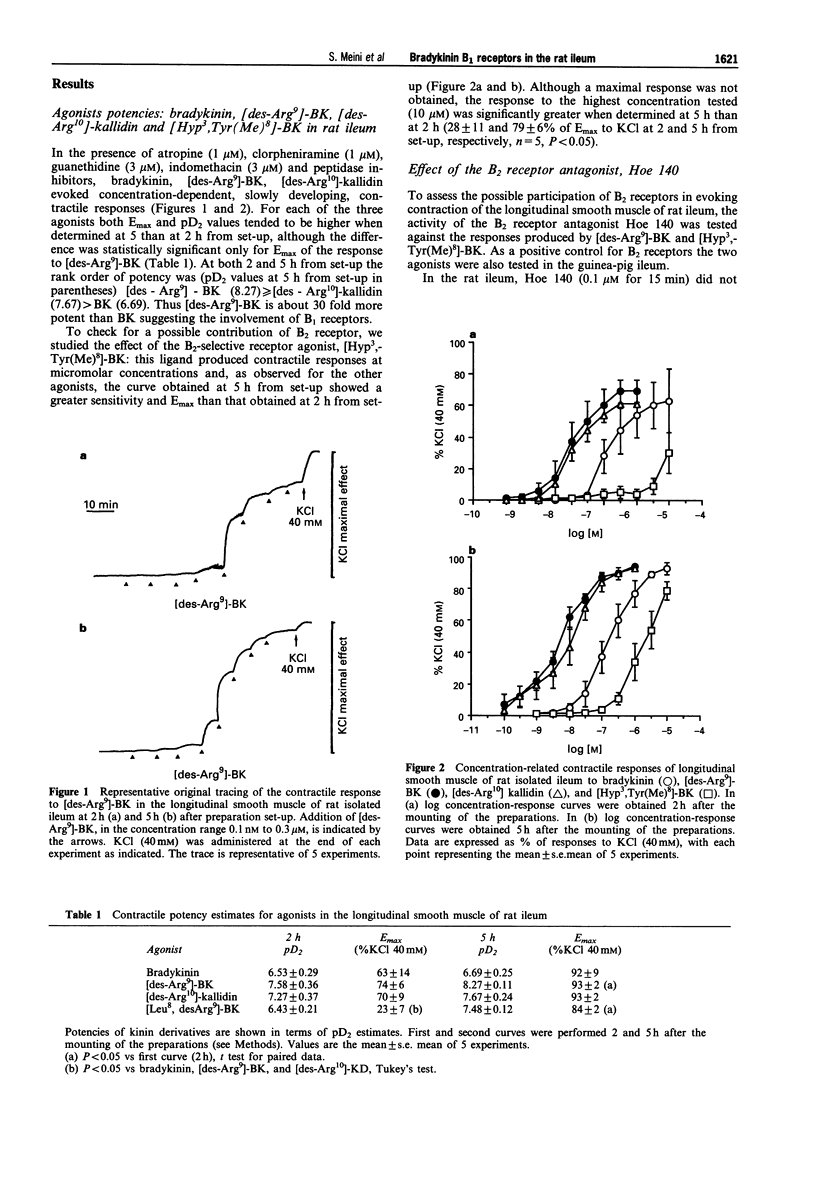
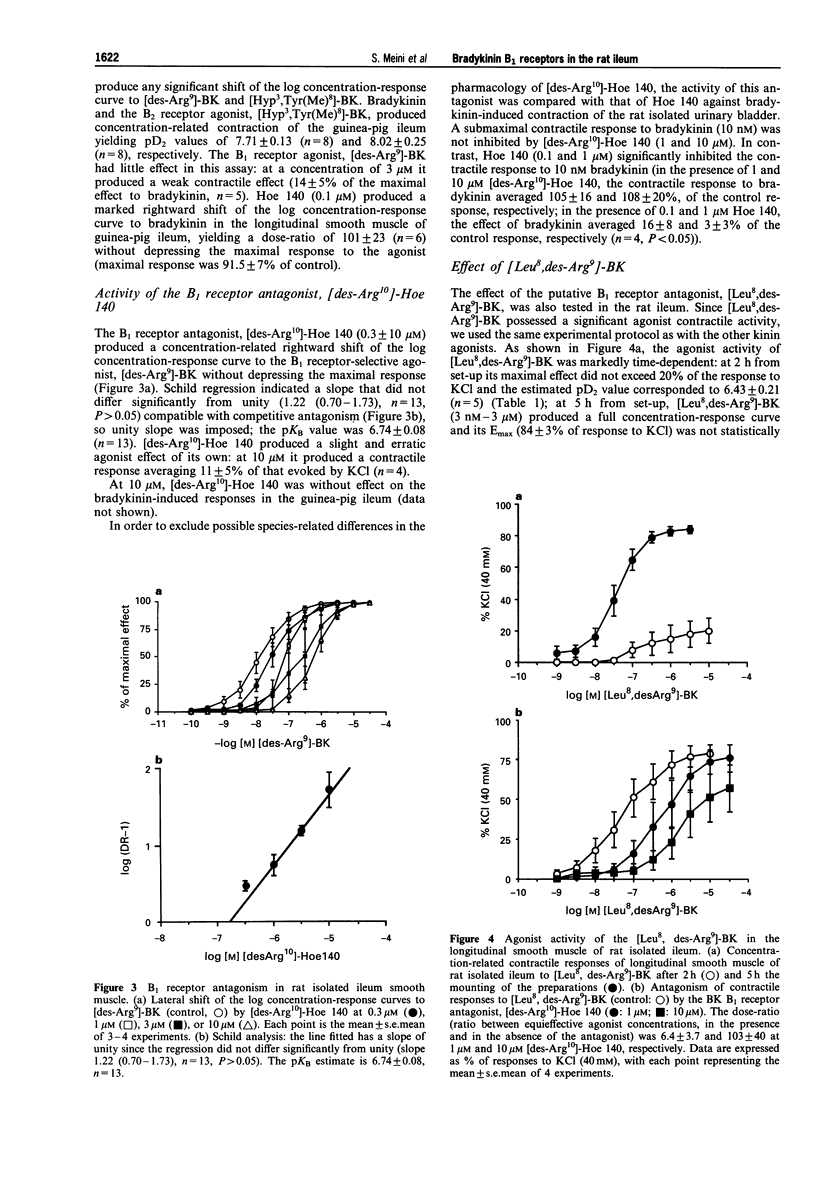
1. Various bradykinin derivatives, acting preferentially at B1 or B2 receptors, were tested in the isolated longitudinal smooth muscle of rat ileum. Experiments were carried out in the presence of chlorpheniramine and atropine (both 1 microM), guanethidine and indomethacin (both 3 microM) and of the peptidase inhibitors (captopril, bestatin and thiorphan, all 1 microM). 2. The rank order of potency was (pD2 values +/- s.e.mean, n = 5 in parentheses, at 5 h from set-up): [des-Arg9]-BK (8.27 +/- 0.11) > or = [des-Arg10]-kallidin (7.67 +/- 0.24) > bradykinin (6.69 +/- 0.25). The B2 receptor selective agonist, [Hyp3,Tyr(Me)8]-BK, was approximately 10 fold less active than bradykinin. Contractile responses to all agonists increased with time. The maximal response to the B1 receptor agonist, [desArg9]-BK at 5 h (94 +/- 2%) was significantly (P < 0.05) greater than that measured at 2 h (74 +/- 2%). 3. The B2 receptor antagonist, D-Arg[Hyp3, Thi5, D-Tic7, Oic8]-BK (Hoe 140, 0.1 microM) did not affect responses to the B1 receptor agonist [des-Arg9]-BK (0.1 nM--1 microM) nor those to the B2 receptor agonist, [Hyp3,Tyr(Me)8]-BK (1 nM--10 microM). In control experiments performed in the longitudinal smooth muscle of guinea-pig ileum and rat isolated urinary bladder as bioassays for B2 receptors, the B2 receptor antagonist Hoe 140 (0.1 microM) antagonized bradykinin-induced contractions. 4. In the rat isolated ileum the B1 receptor antagonist, D-Arg[Hyp3, Thi5, D-Tic7, Oic8, des-Arg9]-BK ([des-Arg10]-Hoe 140, 0.3 - 10 microM) competitively antagonized contractile responses to [des-Arg9]-BK with an estimated pKB of 6.74 +/- 0.08 (Schild plot slope with confidence limits 1.22, (0.70 - 1.73) n = 13). In control experiments in the guinea-pig isolated ileum and rat isolated urinary bladder, [des-Arg10]-Hoe 140 (1 - 10 microM) did not inhibit B2 receptor-mediated contractile responses. 5. The putative B1 receptor antagonist, [Leu8,des-Arg9]-BK, behaved as a partial agonist when responses were determined 2 h from set-up (pD2 6.43 +/- 0.21, n = 5; Emax 30% of that evoked by [des-Arg9]-BK); at 5 h from set-up it behaved as a full agonist (pD2 7.48 +/- 0.12, n = 5; Emax 90% of that evoked by [des-Arg9]-BK). At this time the response to [Leu8,des-Arg9]-BK was antagonized in a concentration-dependent manner by [des-Arg10]-Hoe 140, which at 1 microM and 10 microM, produced dose-ratios of 6.33 +/- 3.66 (n = 4) and 103 +/- 40 (n = 4). 6. In view of the rank order of potency of agonists, the antagonist activity by [des-Arg10]-Hoe 140 and the lack of antagonist activity of Hoe 140, we conclude that the longitudinal smooth muscle of rat ileum, after histamine, acetylcholine, noradrenaline, and prostanoid production blockade, is a sensitive monoreceptor assay for studying the pharmacology of bradykinin B1 receptors. Further the preparation can also be used as a sensitive bioassay to identify partial agonist activity of B1 receptor antagonists such as [Leu8,desArg9]-BK.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó L., Lefebvre R. A. Nitric oxide causes contraction in the rat isolated small intestine. Eur J Pharmacol. 1994 Jun 23;259(1):101–104. doi: 10.1016/0014-2999(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Boschcov P., Paiva A. C., Paiva T. B., Shimuta S. I. Further evidence for the existence of two receptor sites for bradykinin responsible for the diphasic effect in the rat isolated duodenum. Br J Pharmacol. 1984 Oct;83(2):591–600. doi: 10.1111/j.1476-5381.1984.tb16523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthillier J., Deblois D., Marceau F. Studies on the induction of pharmacological responses to des-Arg9-bradykinin in vitro and in vivo. Br J Pharmacol. 1987 Oct;92(2):257–264. doi: 10.1111/j.1476-5381.1987.tb11319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt S. K., Dawson L. G., Hall J. M. Bradykinin B1 receptors in the rabbit urinary bladder: induction of responses, smooth muscle contraction, and phosphatidylinositol hydrolysis. Br J Pharmacol. 1995 Feb;114(3):612–617. doi: 10.1111/j.1476-5381.1995.tb17183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chercuitte F., Beaulieu A. D., Poubelle P., Marceau F. Carboxypeptidase N (kininase I) activity in blood and synovial fluid from patients with arthritis. Life Sci. 1987 Sep 7;41(10):1225–1232. doi: 10.1016/0024-3205(87)90200-1. [DOI] [PubMed] [Google Scholar]
- Davis A. J., Perkins M. N. Induction of B1 receptors in vivo in a model of persistent inflammatory mechanical hyperalgesia in the rat. Neuropharmacology. 1994 Jan;33(1):127–133. doi: 10.1016/0028-3908(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Deblois D., Bouthillier J., Marceau F. Effect of glucocorticoids, monokines and growth factors on the spontaneously developing responses of the rabbit isolated aorta to des-Arg9-bradykinin. Br J Pharmacol. 1988 Apr;93(4):969–977. doi: 10.1111/j.1476-5381.1988.tb11487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A., Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993 Mar;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., McMillan B. A., Meeker S. N., Burch R. M. Induction of vascular smooth muscle bradykinin B1 receptors in vivo during antigen arthritis. Agents Actions. 1991 Sep;34(1-2):191–193. doi: 10.1007/BF01993275. [DOI] [PubMed] [Google Scholar]
- Hall J. M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992 Nov;56(2):131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Borkowski J. A., Young G. S., Strader C. D., Ransom R. W. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- Hock F. J., Wirth K., Albus U., Linz W., Gerhards H. J., Wiemer G., Henke S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br J Pharmacol. 1991 Mar;102(3):769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H. How we describe competitive antagonists: three questions of usage. Trends Pharmacol Sci. 1991 Feb;12(2):53–54. doi: 10.1016/0165-6147(91)90497-g. [DOI] [PubMed] [Google Scholar]
- Kenakin T. P. The classification of drugs and drug receptors in isolated tissues. Pharmacol Rev. 1984 Sep;36(3):165–222. [PubMed] [Google Scholar]
- Maas J., Rae G. A., Huidobro-Toro J. P., Calixto J. B. Characterization of kinin receptors modulating neurogenic contractions of the mouse isolated vas deferens. Br J Pharmacol. 1995 Apr;114(7):1471–1477. doi: 10.1111/j.1476-5381.1995.tb13372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau F., Barabé J., St-Pierre S., Regoli D. Kinin receptors in experimental inflammation. Can J Physiol Pharmacol. 1980 May;58(5):536–542. doi: 10.1139/y80-088. [DOI] [PubMed] [Google Scholar]
- Menke J. G., Borkowski J. A., Bierilo K. K., MacNeil T., Derrick A. W., Schneck K. A., Ransom R. W., Strader C. D., Linemeyer D. L., Hess J. F. Expression cloning of a human B1 bradykinin receptor. J Biol Chem. 1994 Aug 26;269(34):21583–21586. [PubMed] [Google Scholar]
- Paiva A. C., Paiva T. B., Pereira C. C., Shimuta S. I. Selectivity of bradykinin analogues for receptors mediating contraction and relaxation of the rat duodenum. Br J Pharmacol. 1989 Sep;98(1):206–210. doi: 10.1111/j.1476-5381.1989.tb16883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patacchini R., Santicioli P., Astolfi M., Rovero P., Viti G., Maggi C. A. Activity of peptide and non-peptide antagonists at peripheral NK1 receptors. Eur J Pharmacol. 1992 Apr 29;215(1):93–98. doi: 10.1016/0014-2999(92)90613-9. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M. N., Kelly D. Induction of bradykinin B1 receptors in vivo in a model of ultra-violet irradiation-induced thermal hyperalgesia in the rat. Br J Pharmacol. 1993 Dec;110(4):1441–1444. doi: 10.1111/j.1476-5381.1993.tb13982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud D., Baumgarten C. R., Naclerio R. M., Ward P. E. Kinin metabolism in human nasal secretions during experimentally induced allergic rhinitis. J Immunol. 1987 Jan 15;138(2):428–434. [PubMed] [Google Scholar]
- Pruneau D., Luccarini J. M., Robert C., Bélichard P. Induction of kinin B1 receptor-dependent vasoconstriction following balloon catheter injury to the rabbit carotid artery. Br J Pharmacol. 1994 Apr;111(4):1029–1034. doi: 10.1111/j.1476-5381.1994.tb14847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D., Barabé J., Park W. K. Receptors for bradykinin in rabbit aortae. Can J Physiol Pharmacol. 1977 Aug;55(4):855–867. doi: 10.1139/y77-115. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Regoli D., Marceau F., Barabé J. De novo formation of vascular receptors for bradykinin. Can J Physiol Pharmacol. 1978 Aug;56(4):674–677. doi: 10.1139/y78-109. [DOI] [PubMed] [Google Scholar]
- Rhaleb N. E., Dion S., Barabé J., Rouissi N., Jukic D., Drapeau G., Regoli D. Receptors for kinins in isolated arterial vessels of dogs. Eur J Pharmacol. 1989 Mar 29;162(3):419–427. doi: 10.1016/0014-2999(89)90332-4. [DOI] [PubMed] [Google Scholar]
- Rhaleb N. E., Drapeau G., Dion S., Jukic D., Rouissi N., Regoli D. Structure-activity studies on bradykinin and related peptides: agonists. Br J Pharmacol. 1990 Mar;99(3):445–448. doi: 10.1111/j.1476-5381.1990.tb12947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R., Wilson K. A. Prostaglandins and the contractile action of bradykinin on the longitudinal muscle of rat isolated ileum. Br J Pharmacol. 1979 Dec;67(4):527–533. doi: 10.1111/j.1476-5381.1979.tb08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth K., Breipohl G., Stechl J., Knolle J., Henke S., Schölkens B. DesArg9-D-Arg[Hyp3,Thi5,D-Tic7,Oic8]bradykinin (desArg10-[Hoe140]) is a potent bradykinin B1 receptor antagonist. Eur J Pharmacol. 1991 Nov 26;205(2):217–218. doi: 10.1016/0014-2999(91)90824-a. [DOI] [PubMed] [Google Scholar]
- deBlois D., Bouthillier J., Marceau F. Pulse exposure to protein synthesis inhibitors enhances vascular responses to des-Arg9-bradykinin: possible role of interleukin-1. Br J Pharmacol. 1991 May;103(1):1057–1066. doi: 10.1111/j.1476-5381.1991.tb12300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


