Abstract
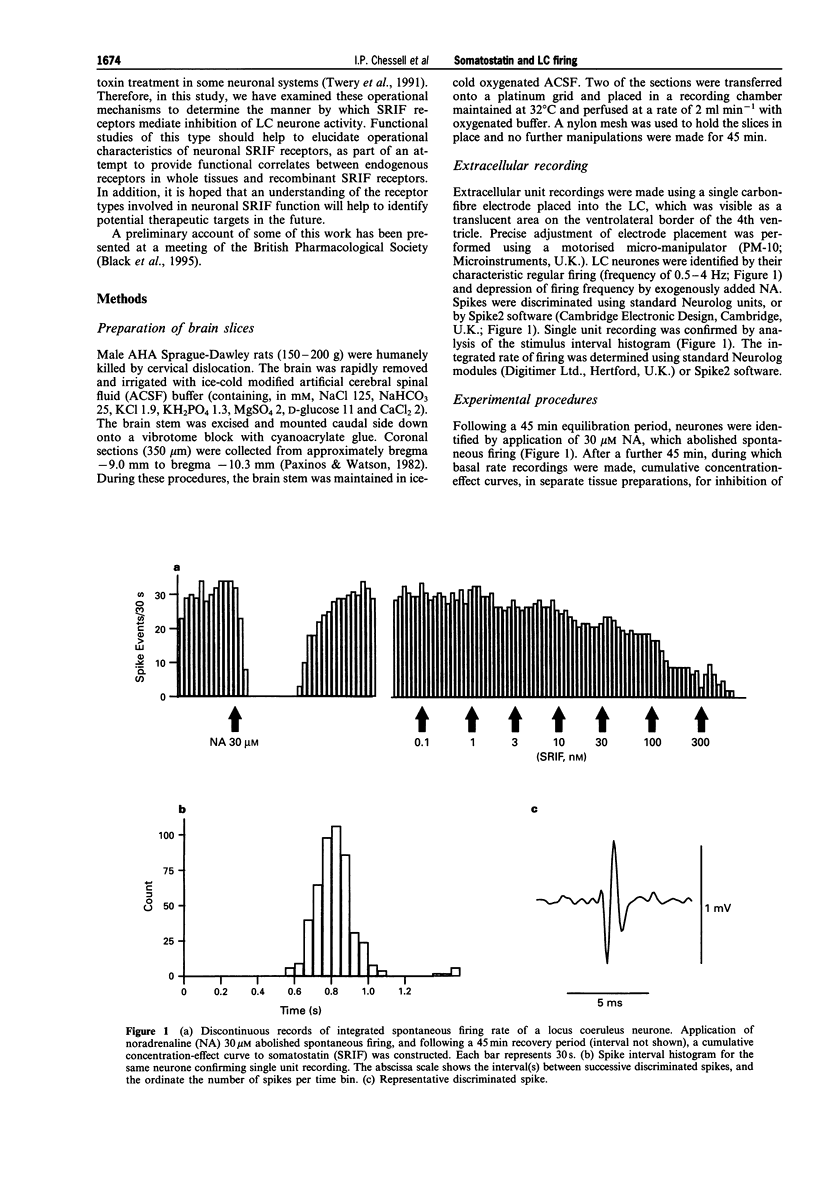
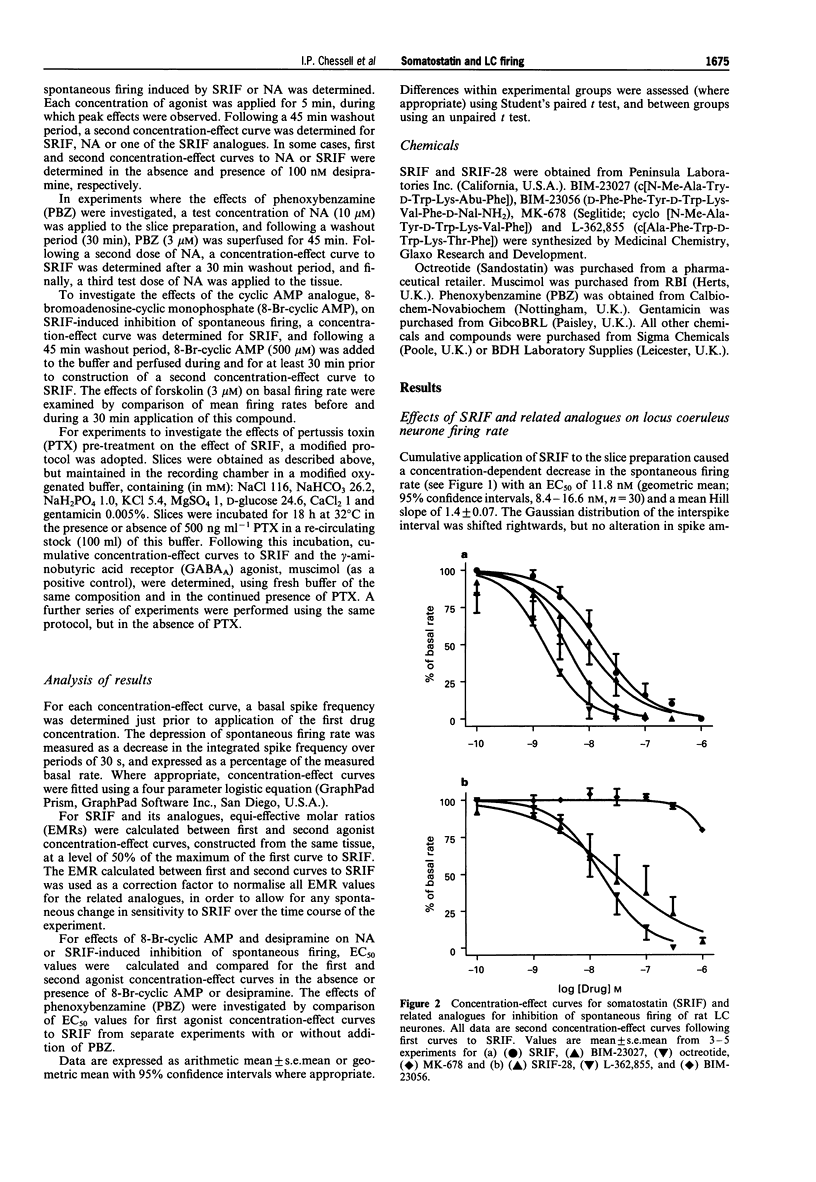
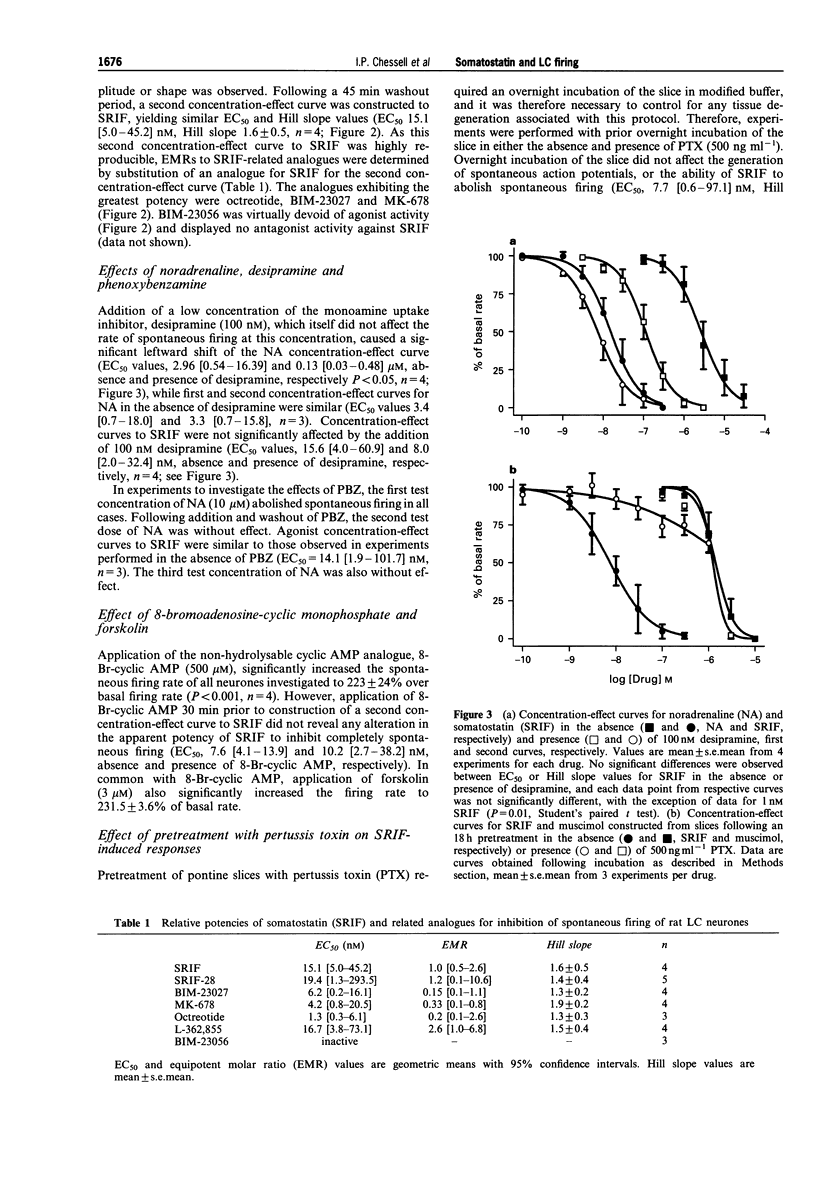
1. In order to characterize somatostatin (SRIF) receptor inhibiting spontaneous firing of rat locus coeruleus neurones, and their transduction mechanism(S), extracellular recordings were obtained from a pontine slice preparation of rat brain containing the locus coeruleus (LC). LC neurones were identified by electrophysiological and pharmacological properties; spontaneous firing (characteristically 0.5-5 Hz) was reversibly and concentration-dependently inhibited by exogenously applied noradrenaline. 2. Spontaneous firing of LC neurones was reversibly and concentration-dependently inhibited by SRIF and the N-terminally extended form, somatostatin-28 (SRIF-28), with EC50 values of 15.1 and 19.4 nM, respectively. The synthetic SRIF analogues (octreotide, MK-678, BIM-23027 and L-362,855) also caused concentration-dependent inhibition of LC neurone firing with a rank order of agonist potencies compatible with actions at a receptor resembling the recombinant sst2 receptor. The putative sst3 selective agonist, BIM-23056, was without agonist or antagonist effect. 3. Addition of 100 nM desipramine significantly increased the efficacy of exogenously applied noradrenaline (EC50 values, 2.96 and 0.13 microM, absence and presence of desipramine, respectively) but did not significantly affect SRIF-induced inhibition (EC50 values, 15.6 and 8.0 nM, respectively). Furthermore, application of phenoxybenzamine (3 microM) abolished responses to NA, but did not affect responses to SRIF (EC50 = 14.1 nM). 4. Application of the cyclic AMP analogue, 8-bromoadenosine-cyclic monophosphate (8-Br-cyclic AMP; 500 microM), significantly increased the spontaneous firing rate of all neurones tested (223 +/- 24% over basal rate). Concentration-effect curves for SRIF constructed in the absence and presence of 8-Br-cyclic AMP had similar threshold concentrations, maxima and EC50 values. 5. Incubation of pontine slices in a modified artificial CSF containing 500 ng ml-1 pertussis toxin (PTX) for 18 h prior to extracellular recording affected neither the spontaneous firing of LC neurones, nor the inhibitory responses to muscimol (EC50 2.2 and 1.2 microM, absence and presence of PTX). However, inhibitory responses to SRIF were markedly attenuated. 6. We conclude that the inhibitory actions of SRIF on spontaneous firing of LC neurones are mediated directly by activation of somatodendritic SRIF receptors, and not indirectly by release of noradrenaline. The SRIF receptors involved appear to couple via a pertussis toxin sensitive G-protein, and elicit their response by a mechanism apparently independent of inhibition of cyclic AMP formation. The agonist profile of several selective and novel SRIF analogues suggests the identity of this receptor to be similar to the recombinant sst2 receptor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Aghajanian G. K. Opiate- and alpha 2-adrenoceptor-induced hyperpolarizations of locus ceruleus neurons in brain slices: reversal by cyclic adenosine 3':5'-monophosphate analogues. J Neurosci. 1985 Sep;5(9):2359–2364. doi: 10.1523/JNEUROSCI.05-09-02359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet M. F., Reisine T. D. Somatostatin regulates dopamine release in rat striatal slices and cat caudate nuclei. J Neurosci. 1983 Jan;3(1):232–236. doi: 10.1523/JNEUROSCI.03-01-00232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T. H., Yeh M. H., Chen M. F. Actions of a long-acting somatostatin analog SMS201-995 (sandostatin) on rat locus coeruleus neurons. Life Sci. 1994;54(18):1313–1320. doi: 10.1016/0024-3205(94)00509-5. [DOI] [PubMed] [Google Scholar]
- Epelbaum J. Somatostatin in the central nervous system: physiology and pathological modifications. Prog Neurobiol. 1986;27(1):63–100. doi: 10.1016/0301-0082(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Feniuk W., Dimech J., Jarvie E. M., Humphrey P. P. Further evidence from functional studies for somatostatin receptor heterogeneity in guinea-pig isolated ileum, vas deferens and right atrium. Br J Pharmacol. 1995 Jul;115(6):975–980. doi: 10.1111/j.1476-5381.1995.tb15906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D., Bell G. I., Berelowitz M., Epelbaum J., Feniuk W., Humphrey P. P., O'Carroll A. M., Patel Y. C., Schonbrunn A., Taylor J. E. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995 Mar;16(3):86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Lübbert H., Bruns C. Molecular pharmacology of somatostatin receptors. Naunyn Schmiedebergs Arch Pharmacol. 1994 Nov;350(5):441–453. doi: 10.1007/BF00173012. [DOI] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama H., Emson P. C. Distribution of somatostatin mRNA in the rat nervous system as visualized by a novel non-radioactive in situ hybridization histochemistry procedure. Neuroscience. 1990;38(1):223–244. doi: 10.1016/0306-4522(90)90388-k. [DOI] [PubMed] [Google Scholar]
- Lachowicz A., Pawlikowski M., Ochedalski T. Somatostatin-14 increases the inositol-1,4,5-trisphosphate content in various areas of the brain. Biochem Biophys Res Commun. 1994 Aug 30;203(1):379–384. doi: 10.1006/bbrc.1994.2193. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Markstein R., Stöckli K. A., Reubi J. C. Differential effects of somatostatin on adenylate cyclase as functional correlate for different brain somatostatin receptor subpopulations. Neurosci Lett. 1989 Sep 25;104(1-2):13–18. doi: 10.1016/0304-3940(89)90321-2. [DOI] [PubMed] [Google Scholar]
- McKeen E. S., Feniuk W., Humphrey P. P. Somatostatin receptors mediating inhibition of basal and stimulated electrogenic ion transport in rat isolated distal colonic mucosa. Naunyn Schmiedebergs Arch Pharmacol. 1995 Oct;352(4):402–411. doi: 10.1007/BF00172777. [DOI] [PubMed] [Google Scholar]
- Moyse E., Beaudet A., Bertherat J., Epelbaum J. Light microscopic radioautographic localization of somatostatin binding sites in the brainstem of the rat. J Chem Neuroanat. 1992 Jan-Feb;5(1):75–84. doi: 10.1016/0891-0618(92)90035-o. [DOI] [PubMed] [Google Scholar]
- North R. A., Williams J. T., Surprenant A., Christie M. J. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panconesi A., Del Bianco P. L., Franchi G., Anselmi B., Andreini R. Somatostatin: peripheral venoconstrictive activity and interaction with monoamines in man. Regul Pept. 1987 Sep;18(5-6):267–276. doi: 10.1016/0167-0115(87)90184-4. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Greenwood M. T., Warszynska A., Panetta R., Srikant C. B. All five cloned human somatostatin receptors (hSSTR1-5) are functionally coupled to adenylyl cyclase. Biochem Biophys Res Commun. 1994 Jan 28;198(2):605–612. doi: 10.1006/bbrc.1994.1088. [DOI] [PubMed] [Google Scholar]
- Raynor K., Murphy W. A., Coy D. H., Taylor J. E., Moreau J. P., Yasuda K., Bell G. I., Reisine T. Cloned somatostatin receptors: identification of subtype-selective peptides and demonstration of high affinity binding of linear peptides. Mol Pharmacol. 1993 Jun;43(6):838–844. [PubMed] [Google Scholar]
- Raynor K., O'Carroll A. M., Kong H., Yasuda K., Mahan L. C., Bell G. I., Reisine T. Characterization of cloned somatostatin receptors SSTR4 and SSTR5. Mol Pharmacol. 1993 Aug;44(2):385–392. [PubMed] [Google Scholar]
- Raynor K., Reisine T. Somatostatin receptors. Crit Rev Neurobiol. 1992;6(4):273–289. [PubMed] [Google Scholar]
- Shefner S. A., Chiu T. H. Adenosine inhibits locus coeruleus neurons: an intracellular study in a rat brain slice preparation. Brain Res. 1986 Feb 26;366(1-2):364–368. doi: 10.1016/0006-8993(86)91320-x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Tsujimoto A. Somatostatin facilitates the serotonin release from rat cerebral cortex, hippocampus and hypothalamus slices. Brain Res. 1981 Mar 9;208(1):219–222. doi: 10.1016/0006-8993(81)90636-3. [DOI] [PubMed] [Google Scholar]
- Tatsumi H., Costa M., Schimerlik M., North R. A. Potassium conductance increased by noradrenaline, opioids, somatostatin, and G-proteins: whole-cell recording from guinea pig submucous neurons. J Neurosci. 1990 May;10(5):1675–1682. doi: 10.1523/JNEUROSCI.10-05-01675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto A., Tanaka S. Stimulatory effect of somatostatin on norepinephrine release from rat brain cortex slices. Life Sci. 1981 Feb 23;28(8):903–910. doi: 10.1016/0024-3205(81)90052-7. [DOI] [PubMed] [Google Scholar]
- Twery M. J., Wong L. A., Gallagher J. P. Somatostatin induced hyperpolarization of septal neurons is not blocked by pertussis toxin. Eur J Pharmacol. 1991 Jan 10;192(2):287–291. doi: 10.1016/0014-2999(91)90054-t. [DOI] [PubMed] [Google Scholar]
- Vanetti M., Vogt G., Höllt V. The two isoforms of the mouse somatostatin receptor (mSSTR2A and mSSTR2B) differ in coupling efficiency to adenylate cyclase and in agonist-induced receptor desensitization. FEBS Lett. 1993 Oct 4;331(3):260–266. doi: 10.1016/0014-5793(93)80349-y. [DOI] [PubMed] [Google Scholar]
- Wang H. L., Reisine T., Dichter M. Somatostatin-14 and somatostatin-28 inhibit calcium currents in rat neocortical neurons. Neuroscience. 1990;38(2):335–342. doi: 10.1016/0306-4522(90)90032-y. [DOI] [PubMed] [Google Scholar]


