Abstract
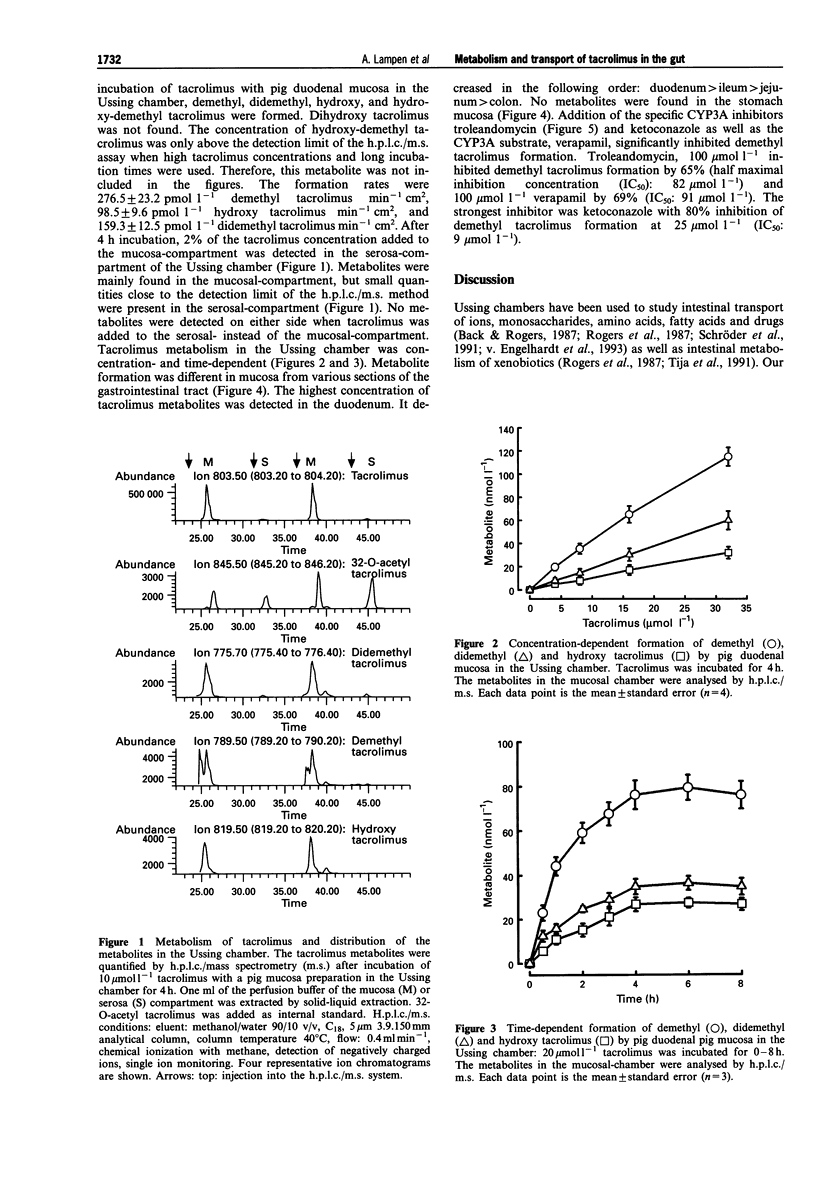
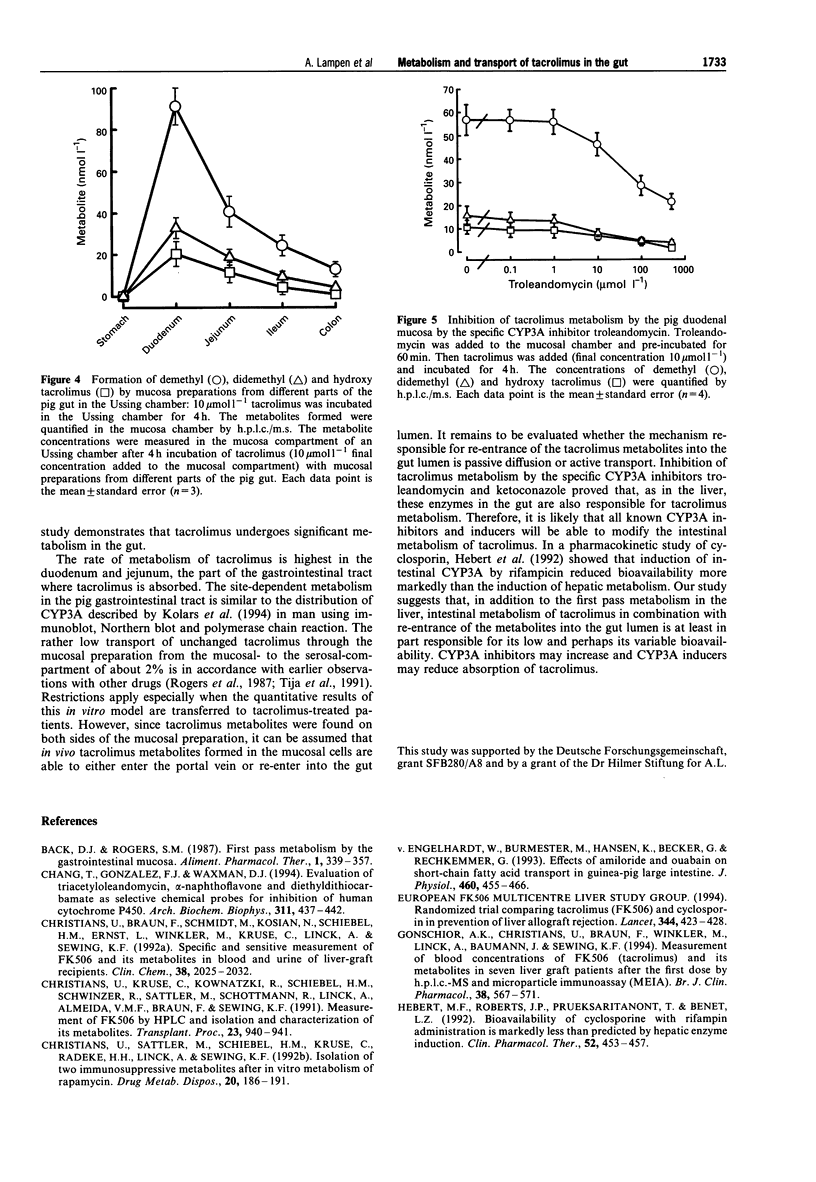
1. The macrolide tacrolimus (FK506), used as an immunosuppressant, is a cytochrome P450 (CYP) 3A substrate in the liver. The metabolism of tacrolimus and the transport of its metabolites in the pig gut was studied in the Ussing chamber. Tacrolimus and its metabolites were quantified by h.p.l.c./mass spectrometry. 2. In the Ussing chamber, demethyl, didemethyl, hydroxy and hydroxy-demethyl tacrolimus were generated. Their formation was concentration- and time-dependent. The metabolite pattern was not different from that after incubation of tacrolimus with human small intestinal microsomes. 3. The metabolite formation was highest in the duodenum and declined in the order duodenum > jejunum > ileum > colon > stomach. 4. Since tacrolimus metabolism was inhibited by the specific CYP3A inhibitors, troleandomycin and ketoconazole, we concluded that these enzymes are involved in intestinal metabolism of tacrolimus. 5. Tacrolimus metabolites re-entered the mucosa chamber (> 90%) and passed through the small intestinal preparation into the serosa chamber. 6. It is concluded that tacrolimus is metabolized in the intestine, that the metabolites are able to re-enter the gut lumen and also enter into the portal vein and that small intestinal metabolism and transport is at least in part responsible for the low oral bioavailability of tacrolimus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. J., Rogers S. M. Review: first-pass metabolism by the gastrointestinal mucosa. Aliment Pharmacol Ther. 1987 Oct;1(5):339–357. doi: 10.1111/j.1365-2036.1987.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Chang T. K., Gonzalez F. J., Waxman D. J. Evaluation of triacetyloleandomycin, alpha-naphthoflavone and diethyldithiocarbamate as selective chemical probes for inhibition of human cytochromes P450. Arch Biochem Biophys. 1994 Jun;311(2):437–442. doi: 10.1006/abbi.1994.1259. [DOI] [PubMed] [Google Scholar]
- Christians U., Braun F., Schmidt M., Kosian N., Schiebel H. M., Ernst L., Winkler M., Kruse C., Linck A., Sewing K. F. Specific and sensitive measurement of FK506 and its metabolites in blood and urine of liver-graft recipients. Clin Chem. 1992 Oct;38(10):2025–2032. [PubMed] [Google Scholar]
- Christians U., Kruse C., Kownatzki R., Schiebel H. M., Schwinzer R., Sattler M., Schottmann R., Linck A., Almeida V. M., Braun F. Measurement of FK 506 by HPLC and isolation and characterization of its metabolites. Transplant Proc. 1991 Feb;23(1 Pt 2):940–941. [PubMed] [Google Scholar]
- Christians U., Sattler M., Schiebel H. M., Kruse C., Radeke H. H., Linck A., Sewing K. F. Isolation of two immunosuppressive metabolites after in vitro metabolism of rapamycin. Drug Metab Dispos. 1992 Mar-Apr;20(2):186–191. [PubMed] [Google Scholar]
- Gonschior A. K., Christians U., Braun F., Winkler M., Linck A., Baumann J., Sewing K. F. Measurement of blood concentrations of FK506 (tacrolimus) and its metabolites in seven liver graft patients after the first dose by h.p.l.c.-MS and microparticle enzyme immunoassay (MEIA). Br J Clin Pharmacol. 1994 Dec;38(6):567–571. doi: 10.1111/j.1365-2125.1994.tb04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert M. F., Roberts J. P., Prueksaritanont T., Benet L. Z. Bioavailability of cyclosporine with concomitant rifampin administration is markedly less than predicted by hepatic enzyme induction. Clin Pharmacol Ther. 1992 Nov;52(5):453–457. doi: 10.1038/clpt.1992.171. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Shiraga T., Matsuda H., Nagase K., Tokuma Y., Hata T., Fujii Y., Sakuma S., Fujitsu T., Fujikawa A. Further metabolism of FK506 (tacrolimus). Identification and biological activities of the metabolites oxidized at multiple sites of FK506. Drug Metab Dispos. 1995 Jan;23(1):28–34. [PubMed] [Google Scholar]
- Iwasaki K., Shiraga T., Nagase K., Tozuka Z., Noda K., Sakuma S., Fujitsu T., Shimatani K., Sato A., Fujioka M. Isolation, identification, and biological activities of oxidative metabolites of FK506, a potent immunosuppressive macrolide lactone. Drug Metab Dispos. 1993 Nov-Dec;21(6):971–977. [PubMed] [Google Scholar]
- Karanam B. V., Vincent S. H., Newton D. J., Wang R. W., Chiu S. H. FK 506 metabolism in human liver microsomes: investigation of the involvement of cytochrome P450 isozymes other than CYP3A4. Drug Metab Dispos. 1994 Sep-Oct;22(5):811–814. [PubMed] [Google Scholar]
- Kolars J. C., Lown K. S., Schmiedlin-Ren P., Ghosh M., Fang C., Wrighton S. A., Merion R. M., Watkins P. B. CYP3A gene expression in human gut epithelium. Pharmacogenetics. 1994 Oct;4(5):247–259. doi: 10.1097/00008571-199410000-00003. [DOI] [PubMed] [Google Scholar]
- Kolars J. C., Stetson P. L., Rush B. D., Ruwart M. J., Schmiedlin-Ren P., Duell E. A., Voorhees J. J., Watkins P. B. Cyclosporine metabolism by P450IIIA in rat enterocytes--another determinant of oral bioavailability? Transplantation. 1992 Mar;53(3):596–602. doi: 10.1097/00007890-199203000-00021. [DOI] [PubMed] [Google Scholar]
- Kroemer H. K., Gautier J. C., Beaune P., Henderson C., Wolf C. R., Eichelbaum M. Identification of P450 enzymes involved in metabolism of verapamil in humans. Naunyn Schmiedebergs Arch Pharmacol. 1993 Sep;348(3):332–337. doi: 10.1007/BF00169164. [DOI] [PubMed] [Google Scholar]
- Lhöest G. J., Maton N., Latinne D., Laurent A., Verbeeck R. K. 15-Desmethyl FK-506 and 15,31-desmethyl FK-506 from human liver microsomes: isolation, identification (by fast atom bombardment mass spectrometry and NMR), and evaluation of in vitro immunosuppressive activity. Clin Chem. 1994 May;40(5):740–744. [PubMed] [Google Scholar]
- Peters D. H., Fitton A., Plosker G. L., Faulds D. Tacrolimus. A review of its pharmacology, and therapeutic potential in hepatic and renal transplantation. Drugs. 1993 Oct;46(4):746–794. doi: 10.2165/00003495-199346040-00009. [DOI] [PubMed] [Google Scholar]
- Pinkus L. M. Separation and use of enterocytes. Methods Enzymol. 1981;77:154–162. doi: 10.1016/s0076-6879(81)77020-4. [DOI] [PubMed] [Google Scholar]
- Porteous J. W., Furneaux H. M., Pearson C. K., Lake C. M., Morrison A. Poly(adenosine diphosphate ribose) synthetase activity in nuclei of dividing and of non-dividing but differentiating intestinal epithelial cells. Biochem J. 1979 Jun 15;180(3):455–461. doi: 10.1042/bj1800455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. M., Back D. J., Orme M. L. Intestinal metabolism of ethinyloestradiol and paracetamol in vitro: studies using Ussing chambers. Br J Clin Pharmacol. 1987 Jun;23(6):727–734. doi: 10.1111/j.1365-2125.1987.tb03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M., Guengerich F. P., Yun C. H., Christians U., Sewing K. F. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992 Sep-Oct;20(5):753–761. [PubMed] [Google Scholar]
- Schröder B., Kaune R., Harmeyer J. Effects of calcitriol on stimulation of ion transport in pig jejunal mucosa. J Physiol. 1991 Feb;433:451–465. doi: 10.1113/jphysiol.1991.sp018437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraga T., Matsuda H., Nagase K., Iwasaki K., Noda K., Yamazaki H., Shimada T., Funae Y. Metabolism of FK506, a potent immunosuppressive agent, by cytochrome P450 3A enzymes in rat, dog and human liver microsomes. Biochem Pharmacol. 1994 Feb 11;47(4):727–735. doi: 10.1016/0006-2952(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Tjia J. F., Webber I. R., Back D. J. Cyclosporin metabolism by the gastrointestinal mucosa. Br J Clin Pharmacol. 1991 Mar;31(3):344–346. doi: 10.1111/j.1365-2125.1991.tb05540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Vincent S. H., Karanam B. V., Painter S. K., Chiu S. H. In vitro metabolism of FK-506 in rat, rabbit, and human liver microsomes: identification of a major metabolite and of cytochrome P450 3A as the major enzymes responsible for its metabolism. Arch Biochem Biophys. 1992 May 1;294(2):454–460. doi: 10.1016/0003-9861(92)90711-5. [DOI] [PubMed] [Google Scholar]
- Wallemacq P. E., Reding R. FK506 (tacrolimus), a novel immunosuppressant in organ transplantation: clinical, biomedical, and analytical aspects. Clin Chem. 1993 Nov;39(11 Pt 1):2219–2228. [PMC free article] [PubMed] [Google Scholar]
- Watkins P. B., Wrighton S. A., Schuetz E. G., Molowa D. T., Guzelian P. S. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987 Oct;80(4):1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C. H., Lee H. S., Lee H., Rho J. K., Jeong H. G., Guengerich F. P. Oxidation of the angiotensin II receptor antagonist losartan (DuP 753) in human liver microsomes. Role of cytochrome P4503A(4) in formation of the active metabolite EXP3174. Drug Metab Dispos. 1995 Feb;23(2):285–289. [PubMed] [Google Scholar]
- von Engelhardt W., Burmester M., Hansen K., Becker G., Rechkemmer G. Effects of amiloride and ouabain on short-chain fatty acid transport in guinea-pig large intestine. J Physiol. 1993 Jan;460:455–466. doi: 10.1113/jphysiol.1993.sp019481. [DOI] [PMC free article] [PubMed] [Google Scholar]


