Abstract
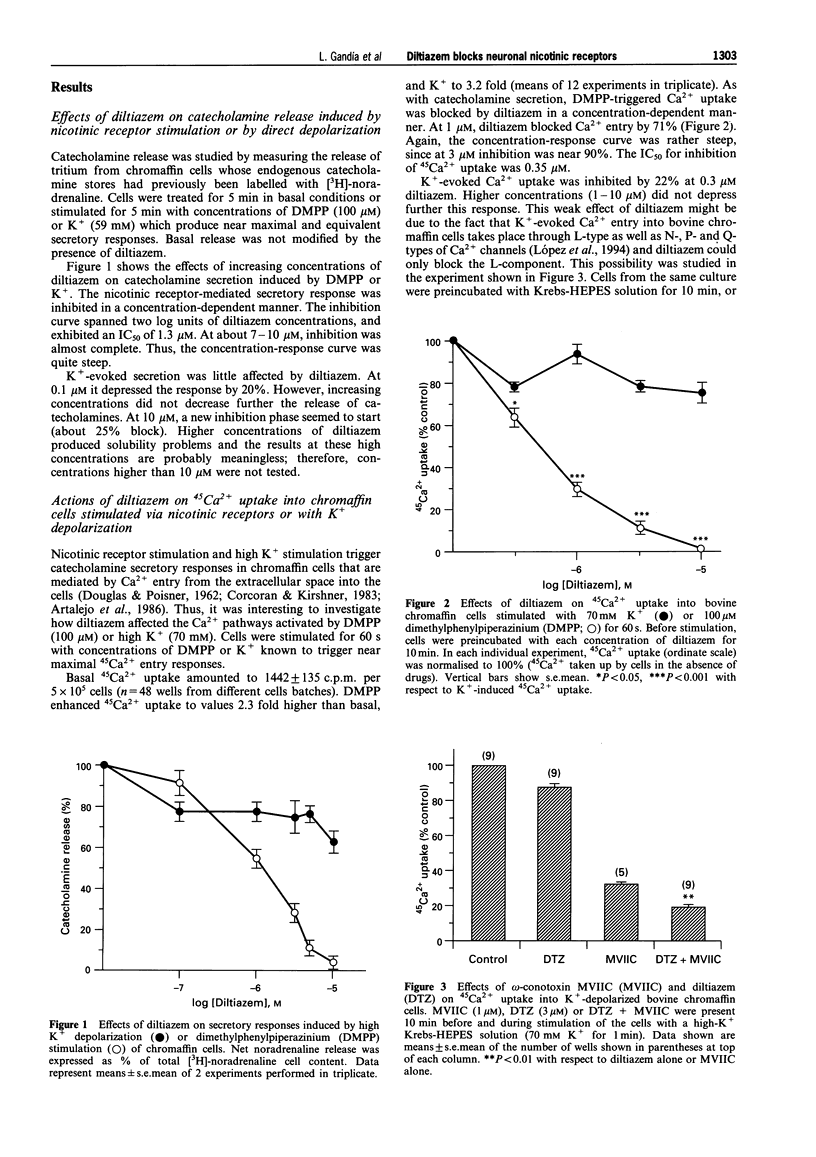
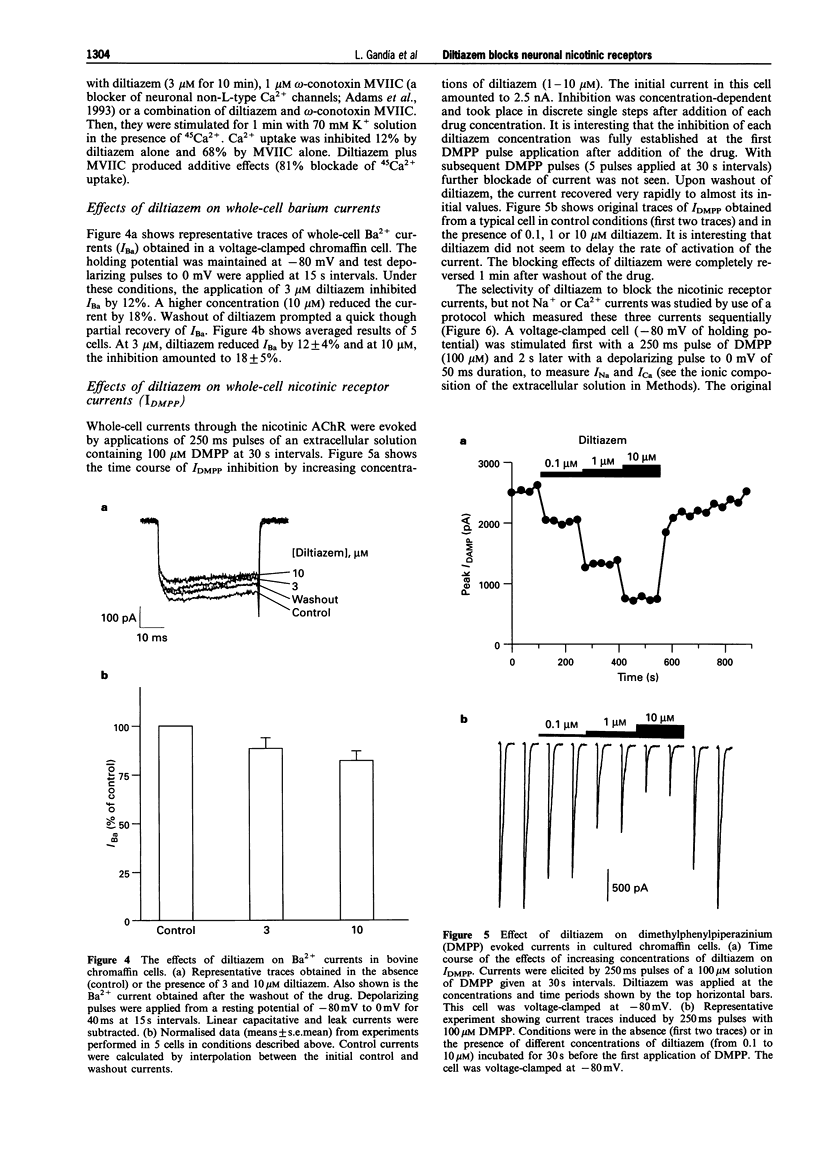
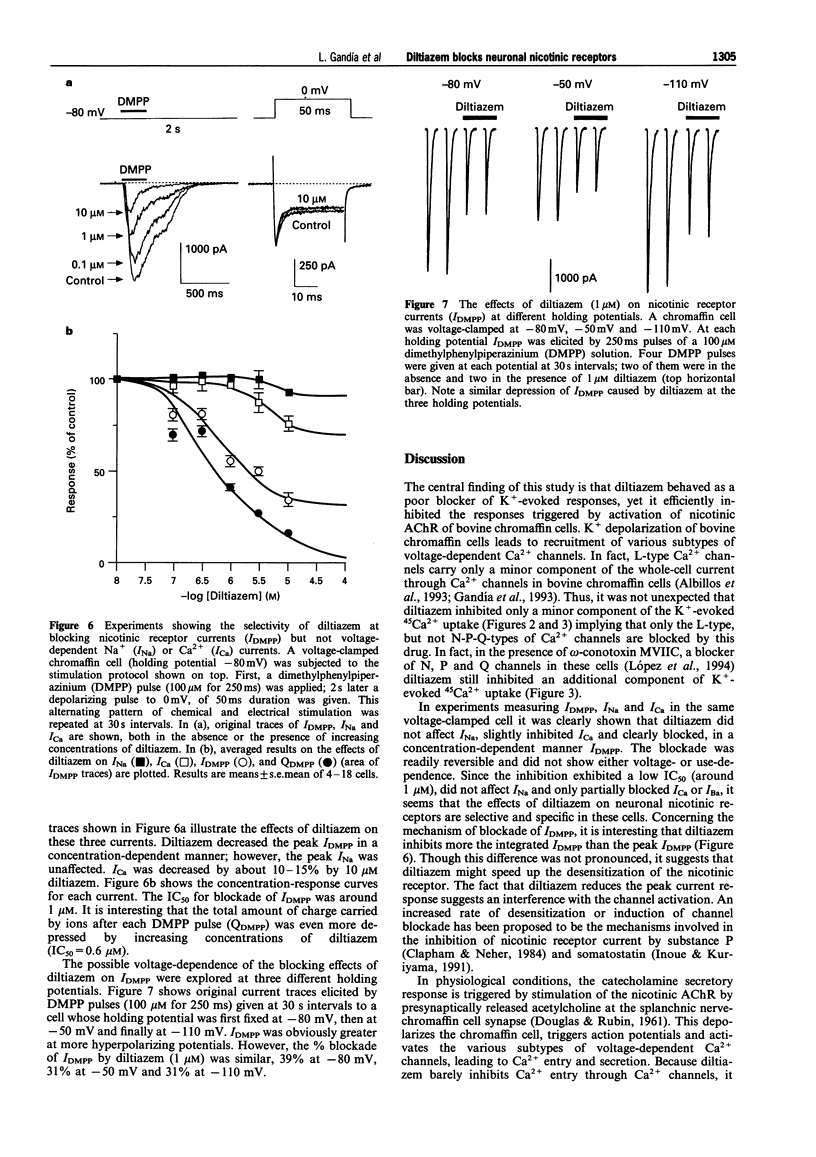
1. The effects of diltiazem on various functional parameters were studied in bovine cultured adrenal chromaffin cells stimulated with the nicotinic receptor agonist dimethylphenylpiperazinium (DMPP) or with depolarizing Krebs-HEPES solutions containing high K+ concentrations. 2. The release of [3H]-noradrenaline induced by DMPP (100 microM for 5 min) was gradually and fully inhibited by increasing concentrations of diltiazem (IC50 = 1.3 microM). In contrast, the highest concentration of diltiazem used (10 microM) inhibited the response to high K+ (59 mM for 5 min) by only 25%. 3. 45Ca2+ uptake into cells stimulated with DMPP (100 microM for 1 min) was also blocked by diltiazem in a concentration-dependent manner (IC50 = 0.4 microM). Again, diltiazem blocked the K(+)-evoked 45Ca2+ uptake (70 mM K+ for 1 min) only by 20%. In contrast, the N-P-Q-type Ca2+ channel blocker omega-conotoxin MVIIC depressed the K+ signal by 70%. In the presence of this toxin, diltiazem exhibited an additional small inhibitory effect, indicating that the compound was acting on L-type Ca2+ channels. 4. Whole-cell Ba2+ currents through Ca2+ channels in voltage-clamped chromaffin cells were inhibited by 3-10 microM diltiazem by 20-25%. The inhibition was readily reversed upon washout of the drug. 5. The whole-cell currents elicited by 100 microM DMPP (IDMPP) were inhibited in a concentration-dependent and reversible manner by diltiazem. Maximal effects were found at 10 microM, which reduced the peak IDMPP by 70%. The area of each curve represented by total current (QDMPP) was reduced more than the peak current. At 10 microM, the inhibition amounted to 80%; the IC50 for QDMPP inhibition was 0.73 microM, a figure close to the IC50 for 45Ca2+ uptake (0.4 microM) and [3H]-noradrenaline release (1.3 microM). The blocking effects of diltiazem developed very quickly and did not exhibit use-dependence; thus the drug blocked the channel in its closed state. The blocking effects of 1 microM diltiazem on IDMPP were similar at different holding potentials (inhibition by around 30% at -100, -80 or -50 mV). Diltiazem did not affect the current flow through voltage-dependent Na+ channels. 6. These data are compatible with the idea that diltiazem has little effect on Ca2+ entry through voltage-dependent Ca2+ channels in bovine chromaffin cells. Neither, does diltiazem affect INa. Rather, diltiazem acts directly on the neuronal nicotinic receptor ion channel and blocks ion fluxes, cell depolarization and the subsequent Ca2+ entry and catecholamine release. This novel effect of diltiazem might have clinical relevance since it might reduce the sympathoadrenal drive to the heart and blood vessels, thus contributing to the well established antihypertensive and cardioprotective effects of the drug.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Myers R. A., Imperial J. S., Olivera B. M. Toxityping rat brain calcium channels with omega-toxins from spider and cone snail venoms. Biochemistry. 1993 Nov 30;32(47):12566–12570. doi: 10.1021/bi00210a003. [DOI] [PubMed] [Google Scholar]
- Albillos A., García A. G., Gandía L. omega-Agatoxin-IVA-sensitive calcium channels in bovine chromaffin cells. FEBS Lett. 1993 Dec 27;336(2):259–262. doi: 10.1016/0014-5793(93)80815-c. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Bader M. F., Aunis D., García A. G. Inactivation of the early calcium uptake and noradrenaline release evoked by potassium in cultured chromaffin cells. Biochem Biophys Res Commun. 1986 Jan 14;134(1):1–7. doi: 10.1016/0006-291x(86)90518-8. [DOI] [PubMed] [Google Scholar]
- Boehm S., Huck S. Methoxyverapamil reduction of nicotine-induced catecholamine release involves inhibition of nicotinic acetylcholine receptor currents. Eur J Neurosci. 1993 Oct 1;5(10):1280–1286. doi: 10.1111/j.1460-9568.1993.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran J. J., Kirshner N. Inhibition of calcium uptake, sodium uptake, and catecholamine secretion by methoxyverapamil (D600) in primary cultures of adrenal medulla cells. J Neurochem. 1983 Apr;40(4):1106–1109. doi: 10.1111/j.1471-4159.1983.tb08099.x. [DOI] [PubMed] [Google Scholar]
- Criado M., Alamo L., Navarro A. Primary structure of an agonist binding subunit of the nicotinic acetylcholine receptor from bovine adrenal chromaffin cells. Neurochem Res. 1992 Mar;17(3):281–287. doi: 10.1007/BF00966671. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A., Kammermeier H., Döring H. J., Freund H. J. Zum Wirkungsmechanismus neuartiger Koronardilatatoren mit gleichzeitig Sauerstoff-einsparenden Myokard-Effekten, Prenylamin und Iproveratril. 1. Z Kreislaufforsch. 1967 Jul;56(7):716–744. [PubMed] [Google Scholar]
- Gandía L., Albillos A., García A. G. Bovine chromaffin cells possess FTX-sensitive calcium channels. Biochem Biophys Res Commun. 1993 Jul 30;194(2):671–676. doi: 10.1006/bbrc.1993.1874. [DOI] [PubMed] [Google Scholar]
- Gandía L., Casado L. F., López M. G., García A. G. Separation of two pathways for calcium entry into chromaffin cells. Br J Pharmacol. 1991 May;103(1):1073–1078. doi: 10.1111/j.1476-5381.1991.tb12302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. S., Boden W. E., Theroux P., Strauss H. D., Pratt C. M., Gheorghiade M., Capone R. J., Crawford M. H., Schlant R. C., Kleiger R. E. Diltiazem and reinfarction in patients with non-Q-wave myocardial infarction. Results of a double-blind, randomized, multicenter trial. N Engl J Med. 1986 Aug 14;315(7):423–429. doi: 10.1056/NEJM198608143150704. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kuriyama H. Somatostatin inhibits the nicotinic receptor-activated inward current in guinea pig chromaffin cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):750–757. doi: 10.1016/0006-291x(91)91481-q. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Slepetis R., Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 1981 Mar;36(3):1245–1255. doi: 10.1111/j.1471-4159.1981.tb01724.x. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- López M. G., Fonteríz R. I., Gandía L., de la Fuente M., Villarroya M., García-Sancho J., García A. G. The nicotinic acetylcholine receptor of the bovine chromaffin cell, a new target for dihydropyridines. Eur J Pharmacol. 1993 Oct 15;247(2):199–207. doi: 10.1016/0922-4106(93)90078-n. [DOI] [PubMed] [Google Scholar]
- López M. G., Villarroya M., Lara B., Martínez Sierra R., Albillos A., García A. G., Gandía L. Q- and L-type Ca2+ channels dominate the control of secretion in bovine chromaffin cells. FEBS Lett. 1994 Aug 8;349(3):331–337. doi: 10.1016/0014-5793(94)00696-2. [DOI] [PubMed] [Google Scholar]
- Moro M. A., López M. G., Gandía L., Michelena P., García A. G. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal Biochem. 1990 Mar;185(2):243–248. doi: 10.1016/0003-2697(90)90287-j. [DOI] [PubMed] [Google Scholar]
- Triggle D. J., Hawthorn M., Gopalakrishnan M., Minarini A., Avery S., Rutledge A., Bangalore R., Zheng W. Synthetic organic ligands active at voltage-gated calcium channels. Ann N Y Acad Sci. 1991;635:123–138. doi: 10.1111/j.1749-6632.1991.tb36487.x. [DOI] [PubMed] [Google Scholar]
- Zernig G. Widening potential for Ca2+ antagonists: non-L-type Ca2+ channel interaction. Trends Pharmacol Sci. 1990 Jan;11(1):38–44. doi: 10.1016/0165-6147(90)90040-f. [DOI] [PubMed] [Google Scholar]


