Abstract
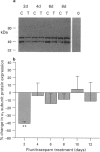
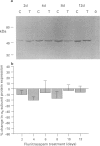
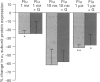
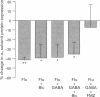
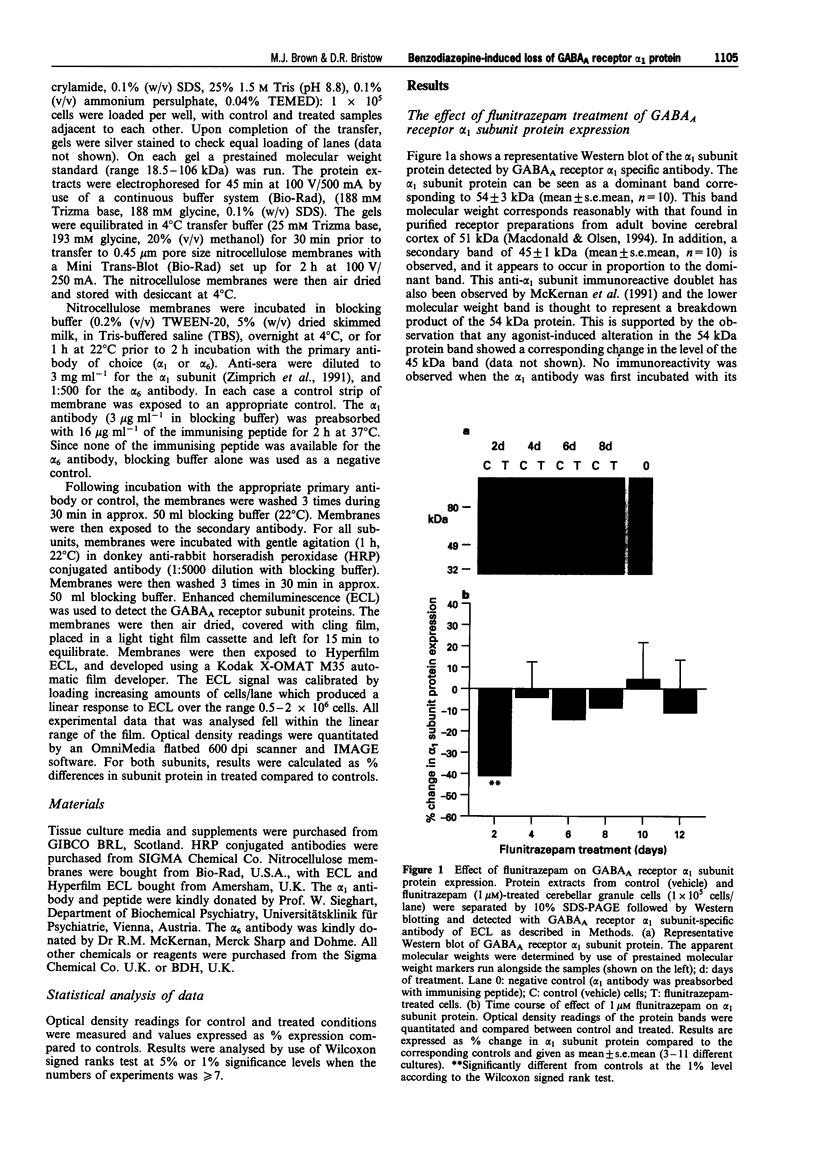
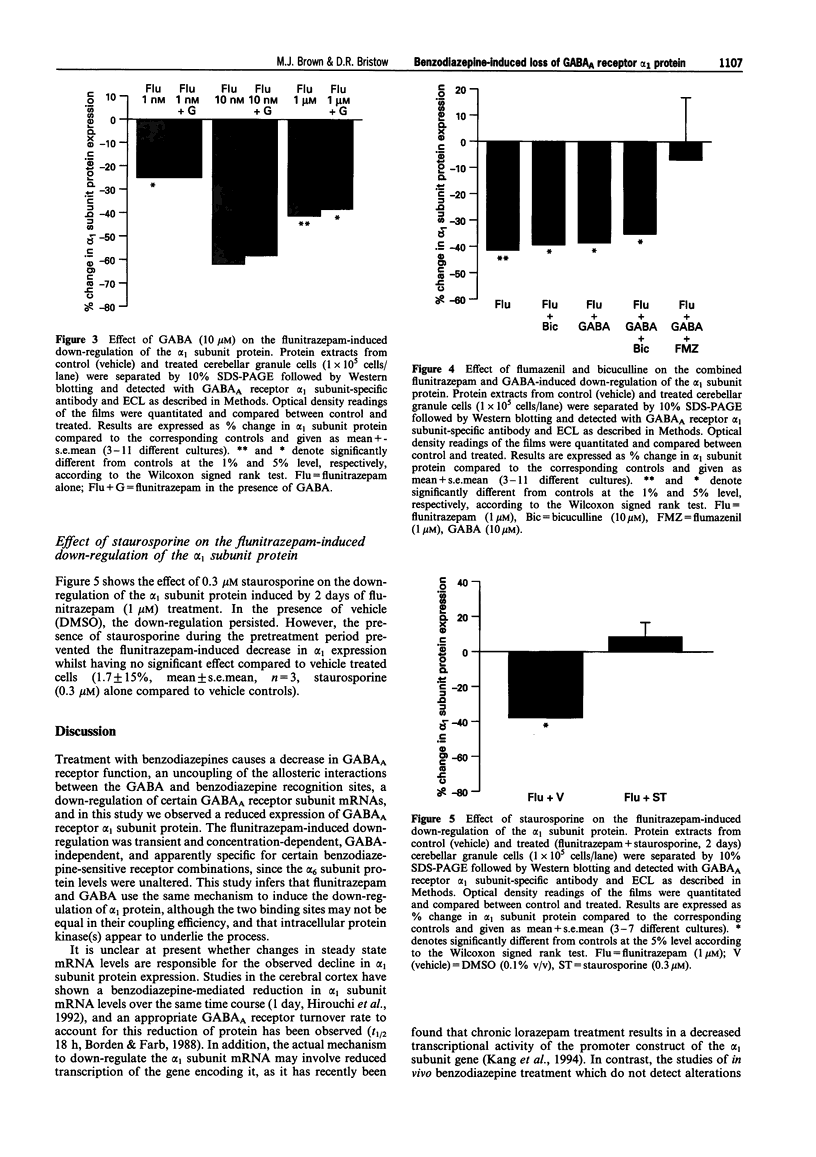
1. Chronic benzodiazepine treatment of rat cerebellar granule cells induced a transient down-regulation of the gamma-aminobutyric acidA (GABAA) receptor alpha 1 subunit protein, that was dose-dependent (1 nM-1 microM) and prevented by the benzodiazepine antagonist flumazenil (1 microM). After 2 days of treatment with 1 microM flunitrazepam the alpha 1 subunit protein was reduced by 41% compared to untreated cells, which returned to, and remained at, control cell levels from 4-12 days of treatment. Chronic flunitrazepam treatment did not significantly alter the GABAA receptor alpha 6 subunit protein over the 2-12 day period. 2. GABA treatment for 2 days down-regulates the alpha 1 subunit protein in a dose-dependent (10 microM-1 mM) manner that was prevented by the selective GABAA receptor antagonist bicuculline (10 microM). At 10 microM and 1 mM GABA the reduction in alpha 1 subunit expression compared to controls was 31% and 66%, respectively. 3. The flunitrazepam-induced decrease in alpha 1 subunit protein is independent of GABA, which suggests that it involves a mechanism distinct from the GABA-dependent action of benzodiazepines on GABAA receptor channel activity. 4. Simultaneous treatment with flunitrazepam and GABA did not produce an additive down-regulation of alpha 1 subunit protein, but produced an effect of the same magnitude as that of flunitrazepam alone. This down-regulation induced by the combination of flunitrazepam and GABA was inhibited by flumazenil (78%), but unaffected by bicuculline. 5. The flunitrazepam-induced down-regulation of alpha 1 subunit protein at 2 days was completely reversed by the protein kinase inhibitor staurosporine (0.3 microM). 6. This study has shown that both flunitrazepam and GABA treatment, via their respective binding sites, caused a reduction in the expression of the GABAA receptor alpha 1 subunit protein; an effect mediated through the same neurochemical mechanism. The results also imply that the benzodiazepine effect is independent of GABA, and that the benzodiazepine and GABA sites may not be equally coupled to the down-regulation process, with the benzodiazepine site being the more dominant. The biochemical mechanism underlying the benzodiazepine-mediated down-regulation of the alpha 1 subunit protein seems to involve the activity of staurosporine-sensitive protein kinases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner B. J., Harvey R. J., Darlison M. G., Barnes E. M., Jr Developmental up-regulation and agonist-dependent down-regulation of GABAA receptor subunit mRNAs in chick cortical neurons. Brain Res Mol Brain Res. 1994 Oct;26(1-2):9–17. doi: 10.1016/0169-328x(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Borden L. A., Farb D. H. Mechanism of gamma-aminobutyric acid/benzodiazepine receptor turnover in neuronal cells: evidence for nonlysosomal degradation. Mol Pharmacol. 1988 Sep;34(3):354–362. [PubMed] [Google Scholar]
- Bristow D. R., Martin I. L. GABA preincubation of rat brain sections increases [3H]GABA binding to the GABAA receptor and compromises the modulatory interactions. Eur J Pharmacol. 1989 Nov 28;173(1):65–73. doi: 10.1016/0014-2999(89)90009-5. [DOI] [PubMed] [Google Scholar]
- Calkin P. A., Barnes E. M., Jr gamma-Aminobutyric acid-A (GABAA) agonists down-regulate GABAA/benzodiazepine receptor polypeptides from the surface of chick cortical neurons. J Biol Chem. 1994 Jan 14;269(2):1548–1553. [PubMed] [Google Scholar]
- Cash D. J., Subbarao K. Desensitization of gamma-aminobutyric acid receptor from rat brain: two distinguishable receptors on the same membrane. Biochemistry. 1987 Dec 1;26(24):7556–7562. doi: 10.1021/bi00398a004. [DOI] [PubMed] [Google Scholar]
- Duggan M. J., Pollard S., Stephenson F. A. Immunoaffinity purification of GABAA receptor alpha-subunit iso-oligomers. Demonstration of receptor populations containing alpha 1 alpha 2, alpha 1 alpha 3, and alpha 2 alpha 3 subunit pairs. J Biol Chem. 1991 Dec 25;266(36):24778–24784. [PubMed] [Google Scholar]
- Gallager D. W., Lakoski J. M., Gonsalves S. F., Rauch S. L. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature. 1984 Mar 1;308(5954):74–77. doi: 10.1038/308074a0. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Tamai K. Coordinate regulation of RNAs encoding two isoforms of the rat muscle nicotinic acetylcholine receptor beta-subunit. Nucleic Acids Res. 1989 Apr 25;17(8):3049–3056. doi: 10.1093/nar/17.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Shader R. I. Dependence, tolerance, and addiction to benzodiazepines: clinical and pharmacokinetic considerations. Drug Metab Rev. 1978;8(1):13–28. doi: 10.3109/03602537808993775. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Tehrani M. H., Barnes E. M., Jr Chronic exposure of developing cortical neurons to GABA down-regulates GABA/benzodiazepine receptors and GABA-gated chloride currents. Brain Res. 1989 Nov 6;501(2):332–338. doi: 10.1016/0006-8993(89)90650-1. [DOI] [PubMed] [Google Scholar]
- Hatten M. E., Liem R. K., Mason C. A. Two forms of cerebellar glial cells interact differently with neurons in vitro. J Cell Biol. 1984 Jan;98(1):193–204. doi: 10.1083/jcb.98.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger C., Gallager D. W. Altered gamma-aminobutyric acid/benzodiazepine interaction after chronic diazepam exposure. Neuropharmacology. 1988 Oct;27(10):1073–1076. doi: 10.1016/0028-3908(88)90070-6. [DOI] [PubMed] [Google Scholar]
- Heninger C., Saito N., Tallman J. F., Garrett K. M., Vitek M. P., Duman R. S., Gallager D. W. Effects of continuous diazepam administration on GABAA subunit mRNA in rat brain. J Mol Neurosci. 1990;2(2):101–107. doi: 10.1007/BF02876917. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- Hirouchi M., Ohkuma S., Kuriyama K. Muscimol-induced reduction of GABAA receptor alpha 1-subunit mRNA in primary cultured cerebral cortical neurons. Brain Res Mol Brain Res. 1992 Oct;15(3-4):327–331. doi: 10.1016/0169-328x(92)90125-u. [DOI] [PubMed] [Google Scholar]
- Hu X. J., Ticku M. K. Chronic benzodiazepine agonist treatment produces functional uncoupling of the gamma-aminobutyric acid-benzodiazepine receptor ionophore complex in cortical neurons. Mol Pharmacol. 1994 Apr;45(4):618–625. [PubMed] [Google Scholar]
- Kang I., Lindquist D. G., Kinane T. B., Ercolani L., Pritchard G. A., Miller L. G. Isolation and characterization of the promoter of the human GABAA receptor alpha 1 subunit gene. J Neurochem. 1994 Apr;62(4):1643–1646. [PubMed] [Google Scholar]
- Kang I., Miller L. G. Decreased GABAA receptor subunit mRNA concentrations following chronic lorazepam administration. Br J Pharmacol. 1991 Jun;103(2):1285–1287. doi: 10.1111/j.1476-5381.1991.tb09781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. L., Whiting P. J., Harris R. A. Benzodiazepine treatment causes uncoupling of recombinant GABAA receptors expressed in stably transfected cells. J Neurochem. 1994 Dec;63(6):2349–2352. doi: 10.1046/j.1471-4159.1994.63062349.x. [DOI] [PubMed] [Google Scholar]
- Krishek B. J., Xie X., Blackstone C., Huganir R. L., Moss S. J., Smart T. G. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994 May;12(5):1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Lüddens H., Wisden W. Function and pharmacology of multiple GABAA receptor subunits. Trends Pharmacol Sci. 1991 Feb;12(2):49–51. doi: 10.1016/0165-6147(91)90495-e. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Mathews G. C., Bolos-Sy A. M., Holland K. D., Isenberg K. E., Covey D. F., Ferrendelli J. A., Rothman S. M. Developmental alteration in GABAA receptor structure and physiological properties in cultured cerebellar granule neurons. Neuron. 1994 Jul;13(1):149–158. doi: 10.1016/0896-6273(94)90465-0. [DOI] [PubMed] [Google Scholar]
- McKernan R. M., Cox P., Gillard N. P., Whiting P. Differential expression of GABAA receptor alpha-subunits in rat brain during development. FEBS Lett. 1991 Jul 29;286(1-2):44–46. doi: 10.1016/0014-5793(91)80936-w. [DOI] [PubMed] [Google Scholar]
- Messer A. The maintenance and identification of mouse cerebellar granule cells in monolayer culture. Brain Res. 1977 Jul 8;130(1):1–12. doi: 10.1016/0006-8993(77)90838-1. [DOI] [PubMed] [Google Scholar]
- Mhatre M. C., Ticku M. K. Chronic GABA treatment downregulates the GABAA receptor alpha 2 and alpha 3 subunit mRNAS as well as polypeptide expression in primary cultured cerebral cortical neurons. Brain Res Mol Brain Res. 1994 Jul;24(1-4):159–165. doi: 10.1016/0169-328x(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Mierlak D., Farb D. H. Modulation of neurotransmitter receptor desensitization: chlordiazepoxide stimulates fading of the GABA response. J Neurosci. 1988 Mar;8(3):814–820. doi: 10.1523/JNEUROSCI.08-03-00814.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. G., Greenblatt D. J., Barnhill J. G., Shader R. I. Chronic benzodiazepine administration. I. Tolerance is associated with benzodiazepine receptor downregulation and decreased gamma-aminobutyric acidA receptor function. J Pharmacol Exp Ther. 1988 Jul;246(1):170–176. [PubMed] [Google Scholar]
- Miller L. G., Roy R. B., Weill C. L. Chronic clonazepam administration decreases gamma-aminobutyric acidA receptor function in cultured cortical neurons. Mol Pharmacol. 1989 Nov;36(5):796–802. [PubMed] [Google Scholar]
- Montpied P., Ginns E. I., Martin B. M., Roca D., Farb D. H., Paul S. M. gamma-Aminobutyric acid (GABA) induces a receptor-mediated reduction in GABAA receptor alpha subunit messenger RNAs in embryonic chick neurons in culture. J Biol Chem. 1991 Apr 5;266(10):6011–6014. [PubMed] [Google Scholar]
- Nayeem N., Green T. P., Martin I. L., Barnard E. A. Quaternary structure of the native GABAA receptor determined by electron microscopic image analysis. J Neurochem. 1994 Feb;62(2):815–818. doi: 10.1046/j.1471-4159.1994.62020815.x. [DOI] [PubMed] [Google Scholar]
- Numann R. E., Wong R. K. Voltage-clamp study on GABA response desensitization in single pyramidal cells dissociated from the hippocampus of adult guinea pigs. Neurosci Lett. 1984 Jun 29;47(3):289–294. doi: 10.1016/0304-3940(84)90528-7. [DOI] [PubMed] [Google Scholar]
- Nutt D. J., Taylor S. C., Little H. J., Standing B. L., Gale R. G. Changes in benzodiazepine/GABA receptor complex function in benzodiazepine-tolerant mice. Psychopharmacology (Berl) 1988;95(3):407–412. doi: 10.1007/BF00181957. [DOI] [PubMed] [Google Scholar]
- O'Donovan M. C., Buckland P. R., Spurlock G., McGuffin P. Bi-directional changes in the levels of messenger RNAs encoding gamma-aminobutyric acidA receptor alpha subunits after flurazepam treatment. Eur J Pharmacol. 1992 Aug 3;226(4):335–341. doi: 10.1016/0922-4106(92)90051-v. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981 Jul;37(1):1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Glaser L., Merlie J. P., Sebanne R., Lindstrom J. Regulation of surface expression of acetylcholine receptors in response to serum and cell growth in the BC3H1 muscle cell line. J Biol Chem. 1983 Nov 25;258(22):13946–13953. [PubMed] [Google Scholar]
- Peter M. G., Davenport A. P. Selectivity of [125I]-PD151242 for human, rat and porcine endothelin ETA receptors in the heart. Br J Pharmacol. 1995 Jan;114(2):297–302. doi: 10.1111/j.1476-5381.1995.tb13226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard S., Duggan M. J., Stephenson F. A. Further evidence for the existence of alpha subunit heterogeneity within discrete gamma-aminobutyric acidA receptor subpopulations. J Biol Chem. 1993 Feb 15;268(5):3753–3757. [PubMed] [Google Scholar]
- Pollard S., Thompson C. L., Stephenson F. A. Quantitative characterization of alpha 6 and alpha 1 alpha 6 subunit-containing native gamma-aminobutyric acidA receptors of adult rat cerebellum demonstrates two alpha subunits per receptor oligomer. J Biol Chem. 1995 Sep 8;270(36):21285–21290. doi: 10.1074/jbc.270.36.21285. [DOI] [PubMed] [Google Scholar]
- Primus R. J., Gallager D. W. GABAA receptor subunit mRNA levels are differentially influenced by chronic FG 7142 and diazepam exposure. Eur J Pharmacol. 1992 May 12;226(1):21–28. doi: 10.1016/0922-4106(92)90078-a. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Roca D. J., Rozenberg I., Farrant M., Farb D. H. Chronic agonist exposure induces down-regulation and allosteric uncoupling of the gamma-aminobutyric acid/benzodiazepine receptor complex. Mol Pharmacol. 1990 Jan;37(1):37–43. [PubMed] [Google Scholar]
- Rosenberg H. C., Chiu T. H. Time course for development of benzodiazepine tolerance and physical dependence. Neurosci Biobehav Rev. 1985 Spring;9(1):123–131. doi: 10.1016/0149-7634(85)90038-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg H. C., Chiu T. H. Tolerance during chronic benzodiazepine treatment associated with decreased receptor binding. Eur J Pharmacol. 1981 Apr 9;70(4):453–460. doi: 10.1016/0014-2999(81)90356-3. [DOI] [PubMed] [Google Scholar]
- Schwartz R. D., Suzdak P. D., Paul S. M. gamma-Aminobutyric acid (GABA)- and barbiturate-mediated 36Cl- uptake in rat brain synaptoneurosomes: evidence for rapid desensitization of the GABA receptor-coupled chloride ion channel. Mol Pharmacol. 1986 Nov;30(5):419–426. [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Rosenberg H. C., Chiu T. H., Zhao T. J. Subunit- and brain region-specific reduction of GABAA receptor subunit mRNAs during chronic treatment of rats with diazepam. J Mol Neurosci. 1994 Summer;5(2):105–120. doi: 10.1007/BF02736752. [DOI] [PubMed] [Google Scholar]
- Zhao T. J., Chiu T. H., Rosenberg H. C. Decreased expression of gamma-aminobutyric acid type A/benzodiazepine receptor beta subunit mRNAs in brain of flurazepam-tolerant rats. J Mol Neurosci. 1994 Fall;5(3):181–192. doi: 10.1007/BF02736732. [DOI] [PubMed] [Google Scholar]
- Zhao T. J., Chiu T. H., Rosenberg H. C. Reduced expression of gamma-aminobutyric acid type A/benzodiazepine receptor gamma 2 and alpha 5 subunit mRNAs in brain regions of flurazepam-treated rats. Mol Pharmacol. 1994 Apr;45(4):657–663. [PubMed] [Google Scholar]
- Zimprich F., Zezula J., Sieghart W., Lassmann H. Immunohistochemical localization of the alpha 1, alpha 2 and alpha 3 subunit of the GABAA receptor in the rat brain. Neurosci Lett. 1991 Jun 10;127(1):125–128. doi: 10.1016/0304-3940(91)90910-l. [DOI] [PubMed] [Google Scholar]