Abstract
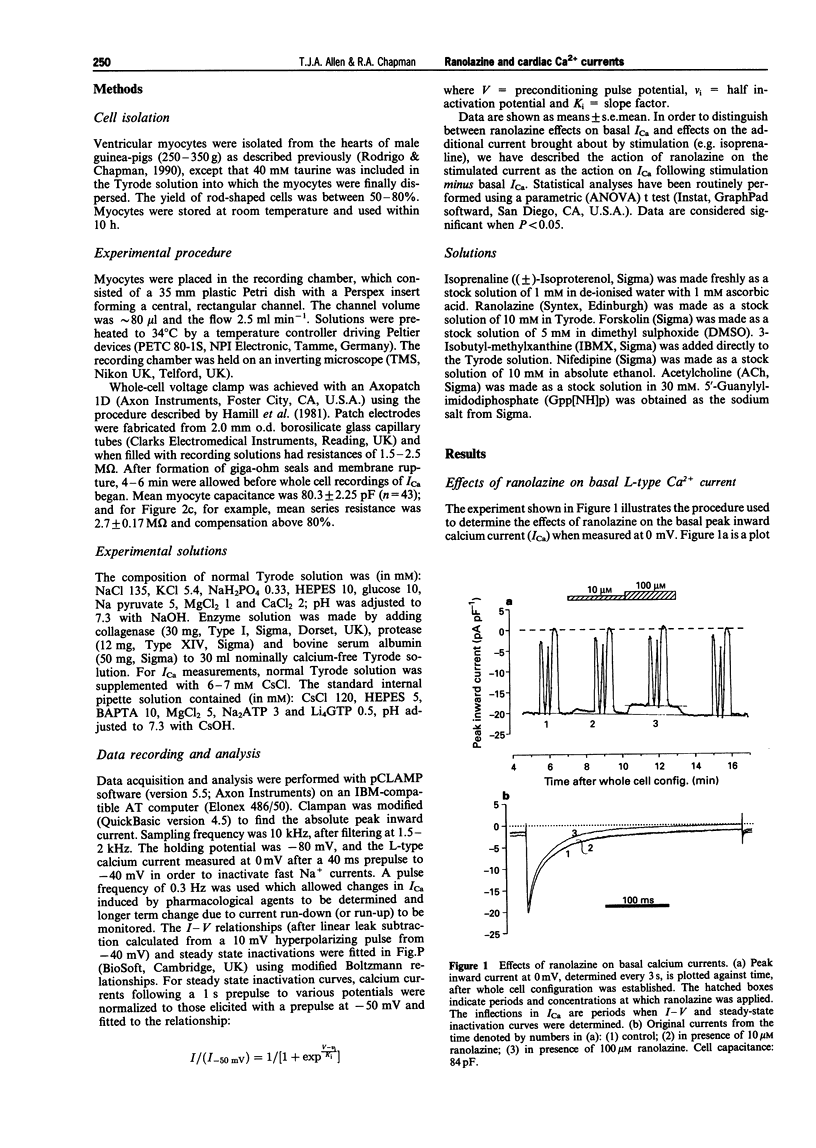
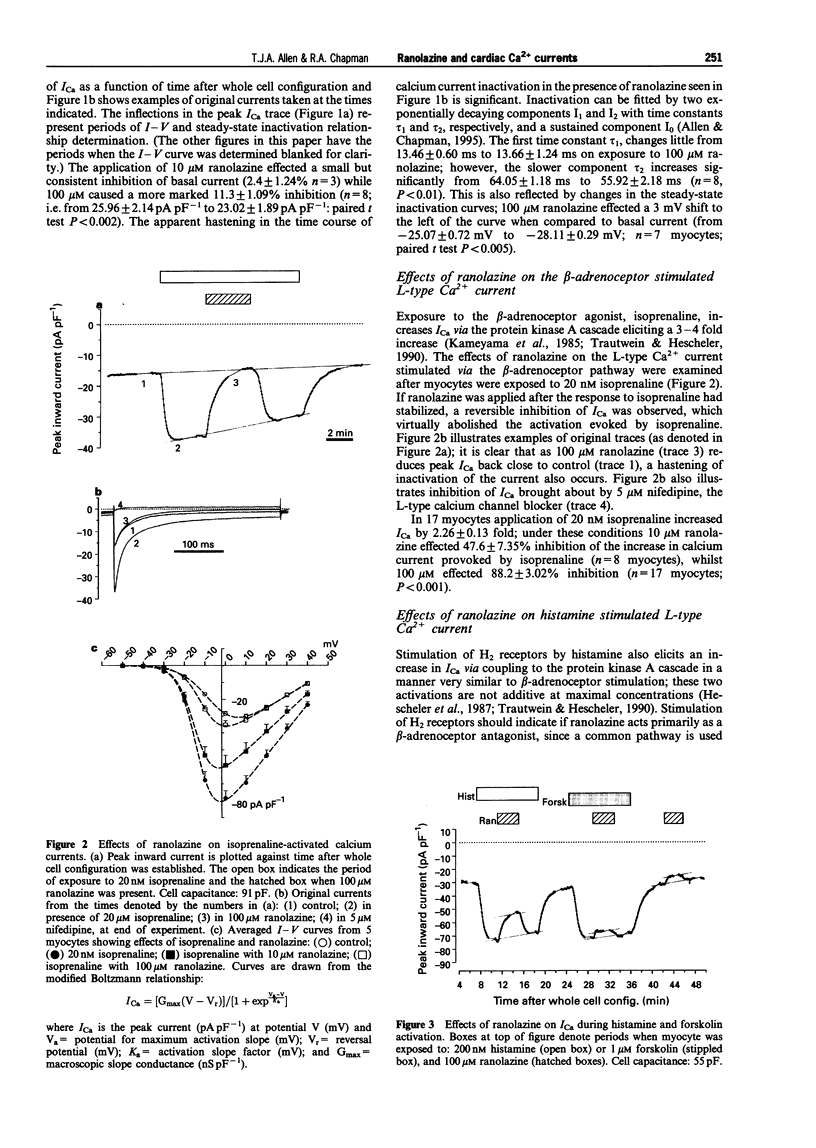
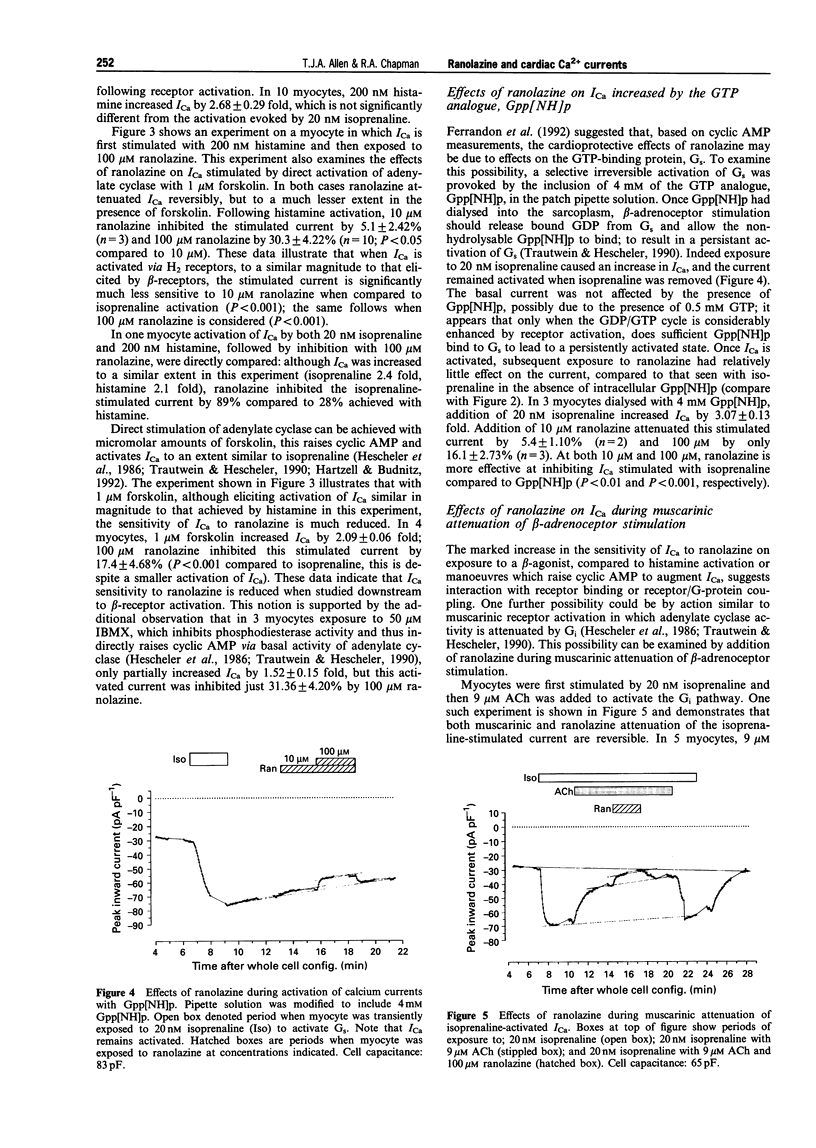
1. Ranolazine has protective effects against ischaemia as exemplified by a reduction of the associated enzyme release and an attenuation of the fall of ATP and other metabolic changes. It has been suggested that ranolazine may affect GTP-binding proteins involved in the beta-adrenergic protein kinase A (PKA) cascade by interacting with Gs. Calcium channel currents are stimulated by this cascade but the effect of ranolazine upon them is not known. The whole cell patch clamp technique was used to examine the action of ranolazine on basal calcium channel currents and those stimulated by activation at various steps in the PKA cascade. 2. Ranolazine had only a small effect on the basal calcium current (100 microM caused 11.3% inhibition), but markedly attenuated the beta-adrenoceptor stimulated current (20 nM isoprenaline increased current by 2.3 fold, 10 microM ranolazine inhibited this increase by 47.6%). When the PKA cascade was activated downstream to the receptor by either G-protein activation with Gpp[NH]p or adenylate cyclase activation with forskolin, the calcium current showed a sensitivity to ranolazine similar to the basal current. Activation of the PKA cascade via H2 receptors gave rise to currents which showed an intermediate sensitivity to ranolazine. Ranolazine inhibition of ICa persisted during muscarinic attenuation of beta-adrenoceptor activation. 3. The results indicate that ranolazine, at concentrations which have significantly beneficial effects during ischaemic episodes, only greatly affects whole cell calcium current when facilitated by beta-adrenoceptor or histamine receptor activation. Ranolazine would appear to act at the receptor level, rather than at the GTP-binding or Gs/adenylate cyclase level. An additional smaller effect is also present, which may be mediated by a direct effect on the channel, or components closely associated with it.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allely M. C., Alps B. J. Prevention of myocardial enzyme release by ranolazine in a primate model of ischaemia with reperfusion. Br J Pharmacol. 1990 Jan;99(1):5–6. doi: 10.1111/j.1476-5381.1990.tb14641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allely M. C., Brown C. M., Kenny B. A., Kilpatrick A. T., Martin A., Spedding M. Modulation of alpha 1-adrenoceptors in rat left ventricle by ischaemia and acyl carnitines: protection by ranolazine. J Cardiovasc Pharmacol. 1993 Jun;21(6):869–873. doi: 10.1097/00005344-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Allen T. J., Chapman R. A. The effect of a chemical phosphatase on single calcium channels and the inactivation of whole-cell calcium current from isolated guinea-pig ventricular myocytes. Pflugers Arch. 1995 May;430(1):68–80. doi: 10.1007/BF00373841. [DOI] [PubMed] [Google Scholar]
- Boutjdir M., Restivo M., Wei Y., el-Sherif N. Alpha 1- and beta-adrenergic interactions on L-type calcium current in cardiac myocytes. Pflugers Arch. 1992 Jul;421(4):397–399. doi: 10.1007/BF00374231. [DOI] [PubMed] [Google Scholar]
- Clarke B., Spedding M., Patmore L., McCormack J. G. Protective effects of ranolazine in guinea-pig hearts during low-flow ischaemia and their association with increases in active pyruvate dehydrogenase. Br J Pharmacol. 1993 Jul;109(3):748–750. doi: 10.1111/j.1476-5381.1993.tb13637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocco G., Rousseau M. F., Bouvy T., Cheron P., Williams G., Detry J. M., Pouleur H. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with beta-blocker or diltiazem. J Cardiovasc Pharmacol. 1992 Jul;20(1):131–138. [PubMed] [Google Scholar]
- Corr P. B., Shayman J. A., Kramer J. B., Kipnis R. J. Increased alpha-adrenergic receptors in ischemic cat myocardium. A potential mediator of electrophysiological derangements. J Clin Invest. 1981 Apr;67(4):1232–1236. doi: 10.1172/JCI110139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski M. R., Black S. C., Kilgore K. S., Chou A. Y., McCormack J. G., Lucchesi B. R. Cardioprotective effects of ranolazine (RS-43285) in the isolated perfused rabbit heart. Cardiovasc Res. 1994 Aug;28(8):1231–1237. doi: 10.1093/cvr/28.8.1231. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Budnitz D. Differences in effects of forskolin and an analog on calcium currents in cardiac myocytes suggest intra- and extracellular sites of action. Mol Pharmacol. 1992 May;41(5):880–888. [PubMed] [Google Scholar]
- Hayashida W., van Eyll C., Rousseau M. F., Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994 Oct;8(5):741–747. doi: 10.1007/BF00877121. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Nawrath H., Tang M., Trautwein W. Adrenoceptor-mediated changes of excitation and contraction in ventricular heart muscle from guinea-pigs and rabbits. J Physiol. 1988 Mar;397:657–670. doi: 10.1113/jphysiol.1988.sp017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Tang M., Jastorff B., Trautwein W. On the mechanism of histamine induced enhancement of the cardiac Ca2+ current. Pflugers Arch. 1987 Sep;410(1-2):23–29. doi: 10.1007/BF00581891. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Maisel A. S., Motulsky H. J., Insel P. A. Externalization of beta-adrenergic receptors promoted by myocardial ischemia. Science. 1985 Oct 11;230(4722):183–186. doi: 10.1126/science.2994229. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Wong T. M., Buja L. M., Lefkowitz R. J., Willerson J. T. Beta adrenergic and muscarinic cholinergic receptors in canine myocardium. Effects of ischemia. J Clin Invest. 1979 Nov;64(5):1423–1428. doi: 10.1172/JCI109600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Gordon M., Stephens D. J., Sturrock W. J. The protective effect of prazosin on the ischaemic and reperfused myocardium. J Mol Cell Cardiol. 1985 Jul;17(7):685–699. doi: 10.1016/s0022-2828(85)80068-7. [DOI] [PubMed] [Google Scholar]
- Rodrigo G. C., Chapman R. A. The calcium paradox in isolated guinea-pig ventricular myocytes: effects of membrane potential and intracellular sodium. J Physiol. 1991 Mar;434:627–645. doi: 10.1113/jphysiol.1991.sp018490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic A., Pucéat M., Clément O., Scamps F., Vassort G. Alpha 1-adrenergic effects on intracellular pH and calcium and on myofilaments in single rat cardiac cells. J Physiol. 1992 Feb;447:275–292. doi: 10.1113/jphysiol.1992.sp019002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadani U., Ezekowitz M., Fenney L., Chiang Y. K. Double-blind efficacy and safety study of a novel anti-ischemic agent, ranolazine, versus placebo in patients with chronic stable angina pectoris. Ranolazine Study Group. Circulation. 1994 Aug;90(2):726–734. doi: 10.1161/01.cir.90.2.726. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Hescheler J. Regulation of cardiac L-type calcium current by phosphorylation and G proteins. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- du Toit E. F., Opie L. H. Modulation of severity of reperfusion stunning in the isolated rat heart by agents altering calcium flux at onset of reperfusion. Circ Res. 1992 May;70(5):960–967. doi: 10.1161/01.res.70.5.960. [DOI] [PubMed] [Google Scholar]


