Abstract
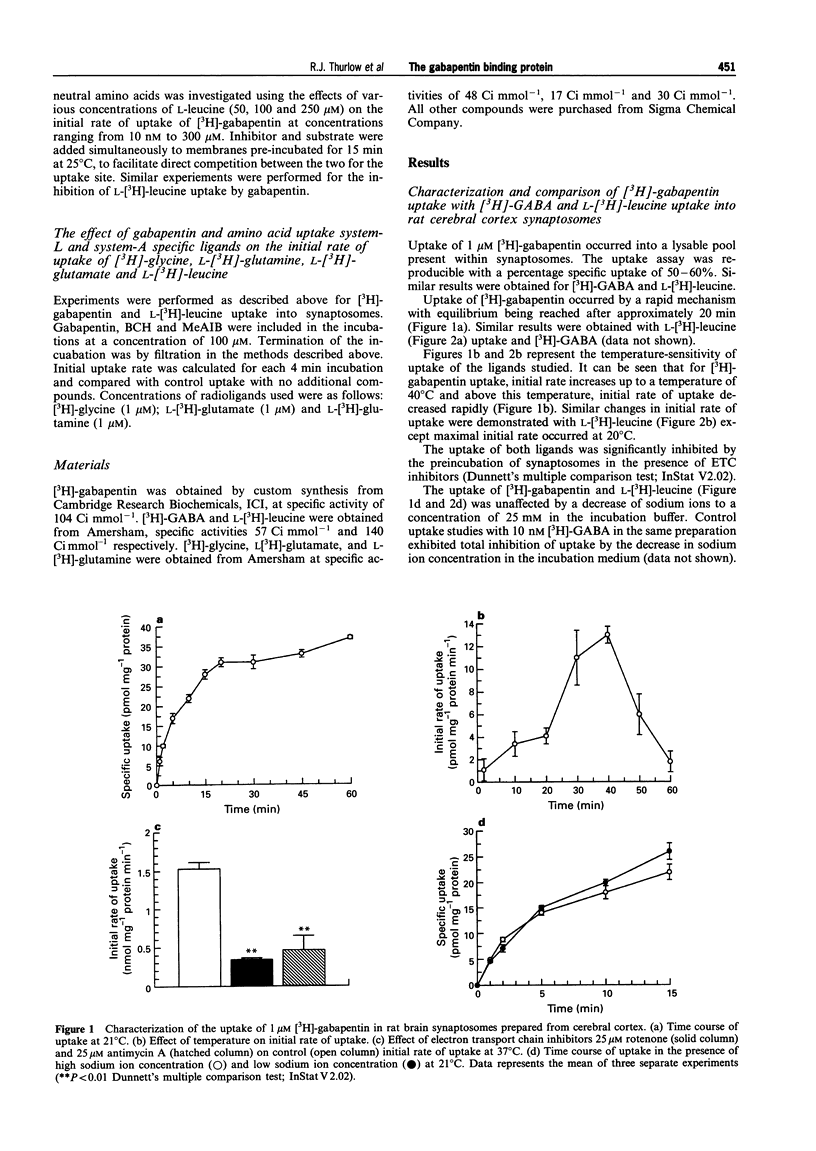
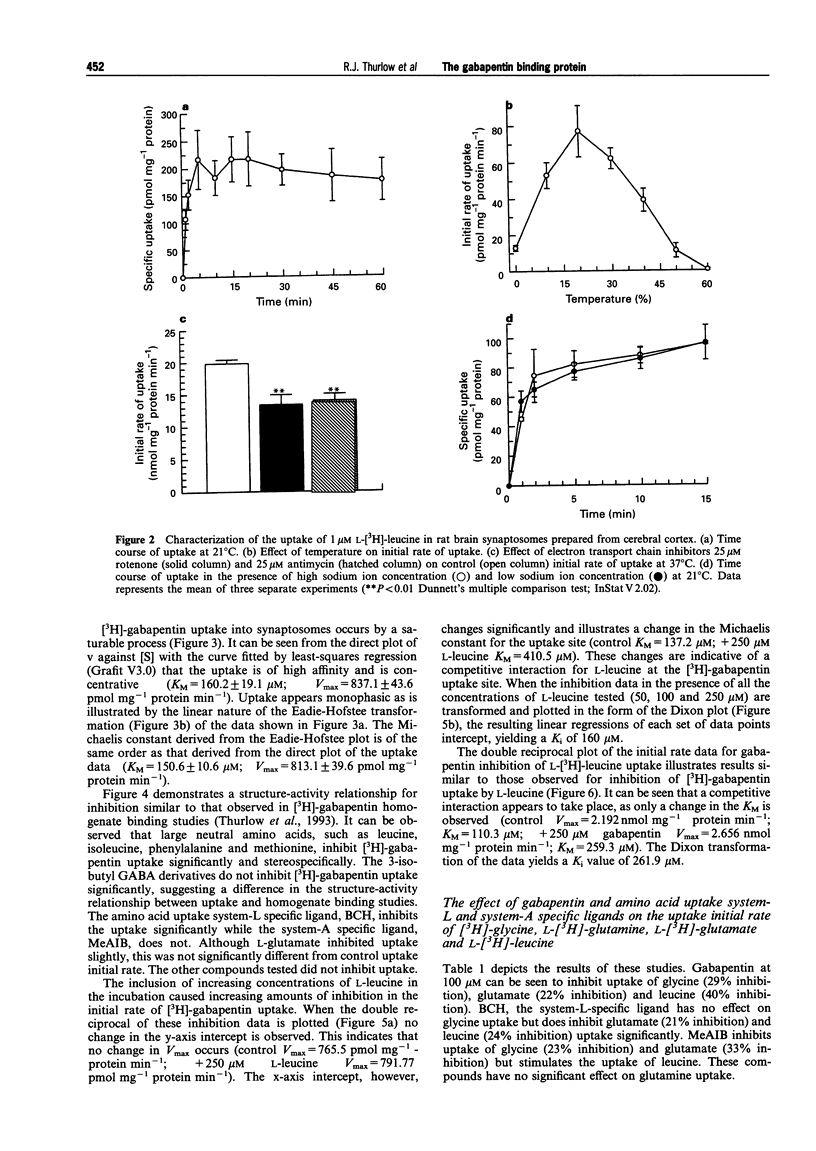
1. Gabapentin is a novel anticonvulsant with an unknown mechanism of action. Homogenate binding studies described elsewhere have suggested that [3H]-gabapentin binds to a site in brain similar to the large neutral amino acid (LNAA) uptake site, termed system-L. 2. This study describes an investigation into the uptake of [3H]-gabapentin into a crude synaptosomal preparation from cerebral cortex of rat brain. Characterization studies showed that [3H]-gabapentin is taken up into synaptosomes by a system that is similar to that responsible for the uptake of L-[3H]-leucine. This system is sodium-independent, temperature-sensitive and requires ATP for function. 3. Kinetic studies of [3H]-gabapentin uptake produced a Michaelis constant (KM = 160 microM) similar to that observed for L-[3H]-leucine (KM = 110.3 microM). Vmax values were 837.1 pmol mg-1 protein min-1 and 2.192 nmol mg-1 protein min-1 respectively. 4. Gabapentin and L-leucine mutually inhibit their uptake. Lineweaver-Burke plots of these data demonstrate that inhibition occurs by a competitive mechanism. Further to this the Dixon transformation of the data illustrates that these two substrates share a common uptake site by the similarity between their calculated Ki and KM values (gabapentin inhibition of L-[3H]-leucine uptake: Ki = 160 microM; L-leucine inhibition of [3H]-gabapentin uptake: Ki = 262 microM). 5. Studies into the effect of gabapentin, the system-L-specific ligand 2-(-)-endoamino-bicycloheptane-2-carboxylic acid (BCH), and the system-A-specific ligand alpha-(methyl-amino)-isobutyric acid (MeAIB), on the initial rate of uptake of [3H]-glycine, L-[3H]-glutamate, L-[3H]-glutamine, and L-[3H]-leucine were performed. At 100 microM, gabapentin significantly inhibited initial rate of uptake of [3H]-glycine (29%), L-[3H]-glutamate (22%) and L-[3H]-leucine (40%). 6. Gabapentin is taken up into synaptosomes by a system similar to system-L, responsible for the uptake of large neutral amino acids. Gabapentin will also inhibit the uptake of certain excitatory amino acids in this synaptosomal preparation. The implications of these findings for the mechanism of action for gabapentin are unclear. The data presented here may suggest an intracellular site for mechanism of action for this compound. Similarly changes in levels of amino acid pools may be involved in the mechanism of gabapentin's anticonvulsant action.
Full text
PDF

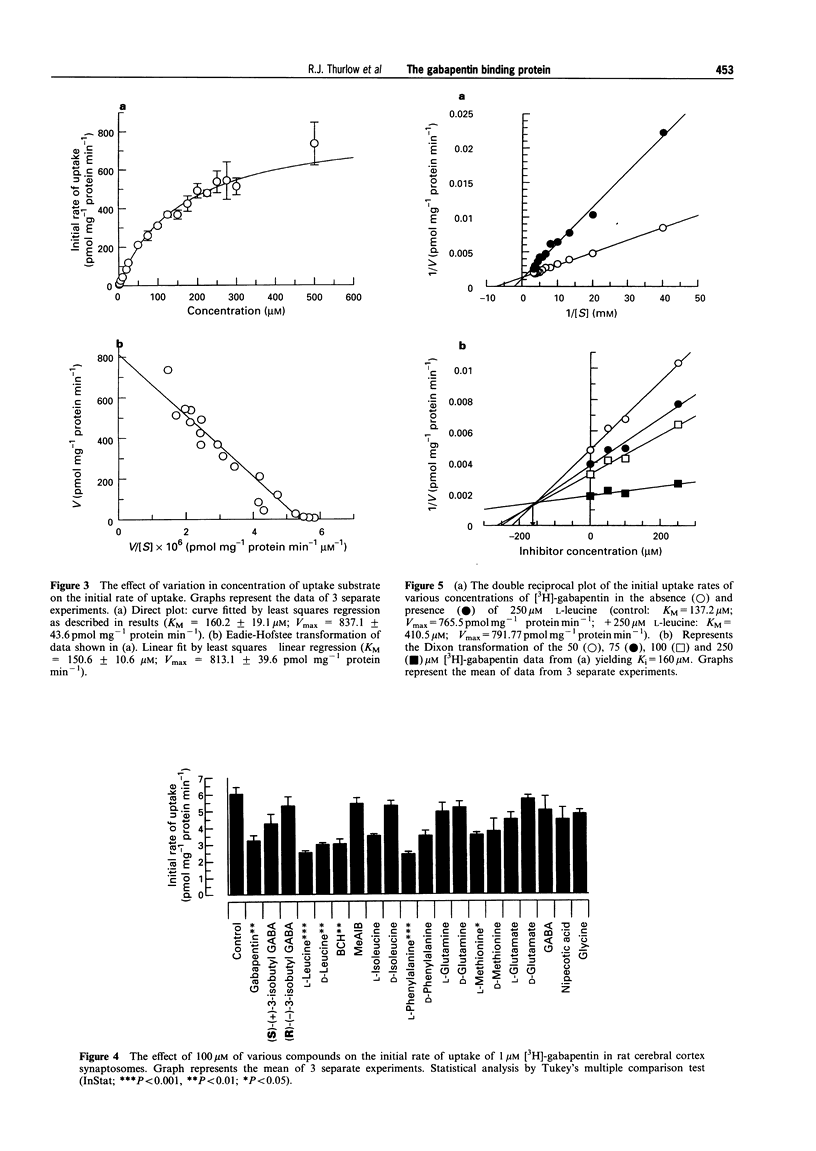
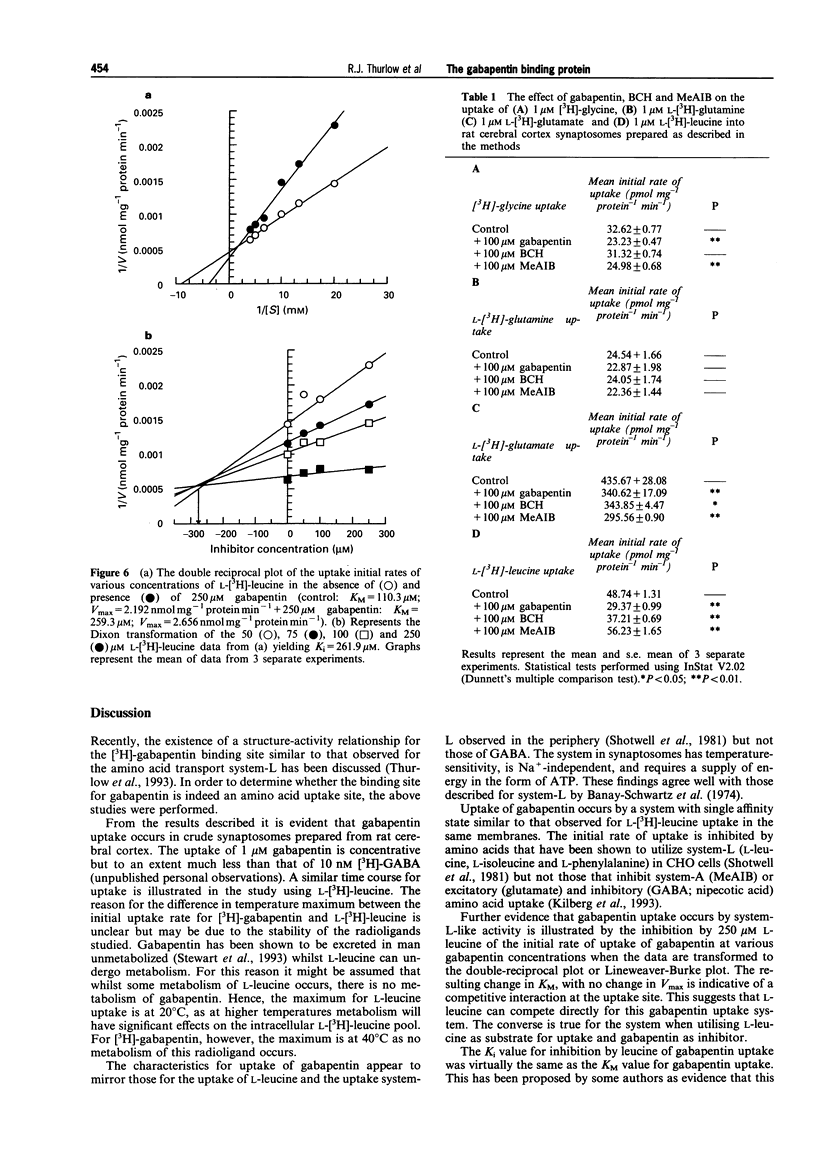


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banay-Schwartz M., Teller D. N., Gergely A., Lajtha A. The effects of metabolic inhibitors on amino acid uptake and the levels of ATP, Na+, and K+ in incubated slices of mouse brain. Brain Res. 1974 May 10;71(1):117–131. doi: 10.1016/0006-8993(74)90195-4. [DOI] [PubMed] [Google Scholar]
- Blasberg R., Lajtha A. Heterogeneity of the mediated transport systems of amino acid uptake in brain. Brain Res. 1966 Jan;1(1):86–104. [PubMed] [Google Scholar]
- CHRISTENSEN H. N., RIGGS T. R., FISCHER H., PALATINE I. M. Amino acid concentration by a free cell neoplasm; relations among amino acids. J Biol Chem. 1952 Sep;198(1):1–15. [PubMed] [Google Scholar]
- Christensen H. N., Handlogten M. E., Lam I., Tager H. S., Zand R. A bicyclic amino acid to improve discriminations among transport systems. J Biol Chem. 1969 Mar 25;244(6):1510–1520. [PubMed] [Google Scholar]
- Christensen H. N. Organic ion transport during seven decades. The amino acids. Biochim Biophys Acta. 1984 Sep 3;779(3):255–269. doi: 10.1016/0304-4157(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Crnic D. M., Hammerstad J. P., Cutler R. W. Accelerated efflux of ( 14 C) and ( 3 H) amino acids from superfused slices of rat brain. J Neurochem. 1973 Jan;20(1):203–209. doi: 10.1111/j.1471-4159.1973.tb12117.x. [DOI] [PubMed] [Google Scholar]
- Denizeau F., Sourkes T. L. Regional transport of tryptophan in rat brain. J Neurochem. 1977 May;28(5):951–959. doi: 10.1111/j.1471-4159.1977.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Parfitt A. G. Tryptophan transport across the synaptosomal membrane. J Neurochem. 1970 Sep;17(9):1339–1353. doi: 10.1111/j.1471-4159.1970.tb06869.x. [DOI] [PubMed] [Google Scholar]
- Hannuniemi R., Oja S. S. Uptake of leucine, lysine, aspartic acid, and glycine into isolated neurons and astrocytes. Neurochem Res. 1981 Aug;6(8):873–884. doi: 10.1007/BF00965045. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Suman-Chauhan N., Woodruff G. N. Localization of [3H]gabapentin to a novel site in rat brain: autoradiographic studies. Eur J Pharmacol. 1993 Feb 15;244(3):303–309. doi: 10.1016/0922-4106(93)90156-4. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Johnston G. A. GABA uptake in rat central nervous system: comparison of uptake in slices and homogenates and the effects of some inhibitors. J Neurochem. 1971 Oct;18(10):1939–1950. doi: 10.1111/j.1471-4159.1971.tb09600.x. [DOI] [PubMed] [Google Scholar]
- Kiely M., Sourkes T. L. Transport of L-tryptophan into slices of rat cerebral cortex. J Neurochem. 1972 Dec;19(12):2863–2872. doi: 10.1111/j.1471-4159.1972.tb03824.x. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Stevens B. R., Novak D. A. Recent advances in mammalian amino acid transport. Annu Rev Nutr. 1993;13:137–165. doi: 10.1146/annurev.nu.13.070193.001033. [DOI] [PubMed] [Google Scholar]
- Korpi E. R. Kinetics of tryptophan influx into brain slices depleted of sodium and loaded with L-histidine. J Neurochem. 1983 Feb;40(2):428–433. doi: 10.1111/j.1471-4159.1983.tb11300.x. [DOI] [PubMed] [Google Scholar]
- Korpi E. R., Oja S. S. Efflux of phenylalanine from rat cerebral cortex slices as influenced by extra- and intracellular amino acids. J Neurochem. 1979 Mar;32(3):789–796. doi: 10.1111/j.1471-4159.1979.tb04562.x. [DOI] [PubMed] [Google Scholar]
- Maragos W. F., Penney J. B., Young A. B. Anatomic correlation of NMDA and 3H-TCP-labeled receptors in rat brain. J Neurosci. 1988 Feb;8(2):493–501. doi: 10.1523/JNEUROSCI.08-02-00493.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivan J. D., Pastor-Anglada M. Regulatory and molecular aspects of mammalian amino acid transport. Biochem J. 1994 Apr 15;299(Pt 2):321–334. doi: 10.1042/bj2990321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Piccoli F., Grynbaum A., Lajtha A. Developmental changes in Na + , K + and ATP and in the levels and transport of amino acids in incubated slices of rat brain. J Neurochem. 1971 Jun;18(6):1135–1148. doi: 10.1111/j.1471-4159.1971.tb12042.x. [DOI] [PubMed] [Google Scholar]
- Sershen H., Lajtha A. Inhibition pattern by analogs indicates the presence of ten or more transport systems for amino acids in brain cells. J Neurochem. 1979 Mar;32(3):719–726. doi: 10.1111/j.1471-4159.1979.tb04554.x. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Jayme D. W., Kilberg M. S., Oxender D. L. Neutral amino acid transport systems in Chinese hamster ovary cells. J Biol Chem. 1981 Jun 10;256(11):5422–5427. [PubMed] [Google Scholar]
- Stewart B. H., Kugler A. R., Thompson P. R., Bockbrader H. N. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res. 1993 Feb;10(2):276–281. doi: 10.1023/a:1018951214146. [DOI] [PubMed] [Google Scholar]
- Su T. Z., Lunney E., Campbell G., Oxender D. L. Transport of gabapentin, a gamma-amino acid drug, by system l alpha-amino acid transporters: a comparative study in astrocytes, synaptosomes, and CHO cells. J Neurochem. 1995 May;64(5):2125–2131. doi: 10.1046/j.1471-4159.1995.64052125.x. [DOI] [PubMed] [Google Scholar]
- Suman-Chauhan N., Webdale L., Hill D. R., Woodruff G. N. Characterisation of [3H]gabapentin binding to a novel site in rat brain: homogenate binding studies. Eur J Pharmacol. 1993 Feb 15;244(3):293–301. doi: 10.1016/0922-4106(93)90155-3. [DOI] [PubMed] [Google Scholar]
- Thurlow R. J., Brown J. P., Gee N. S., Hill D. R., Woodruff G. N. [3H]gabapentin may label a system-L-like neutral amino acid carrier in brain. Eur J Pharmacol. 1993 Nov 15;247(3):341–345. doi: 10.1016/0922-4106(93)90204-m. [DOI] [PubMed] [Google Scholar]
- Vahvelainen M. L., Oja S. S. Kinetics of influx of phenylalanine, tyrosine, tryptophan, histidine and leucine into slices of brain cortex from adult and 7-day-old rats. Brain Res. 1972 May 26;40(2):477–488. doi: 10.1016/0006-8993(72)90148-5. [DOI] [PubMed] [Google Scholar]


