Abstract
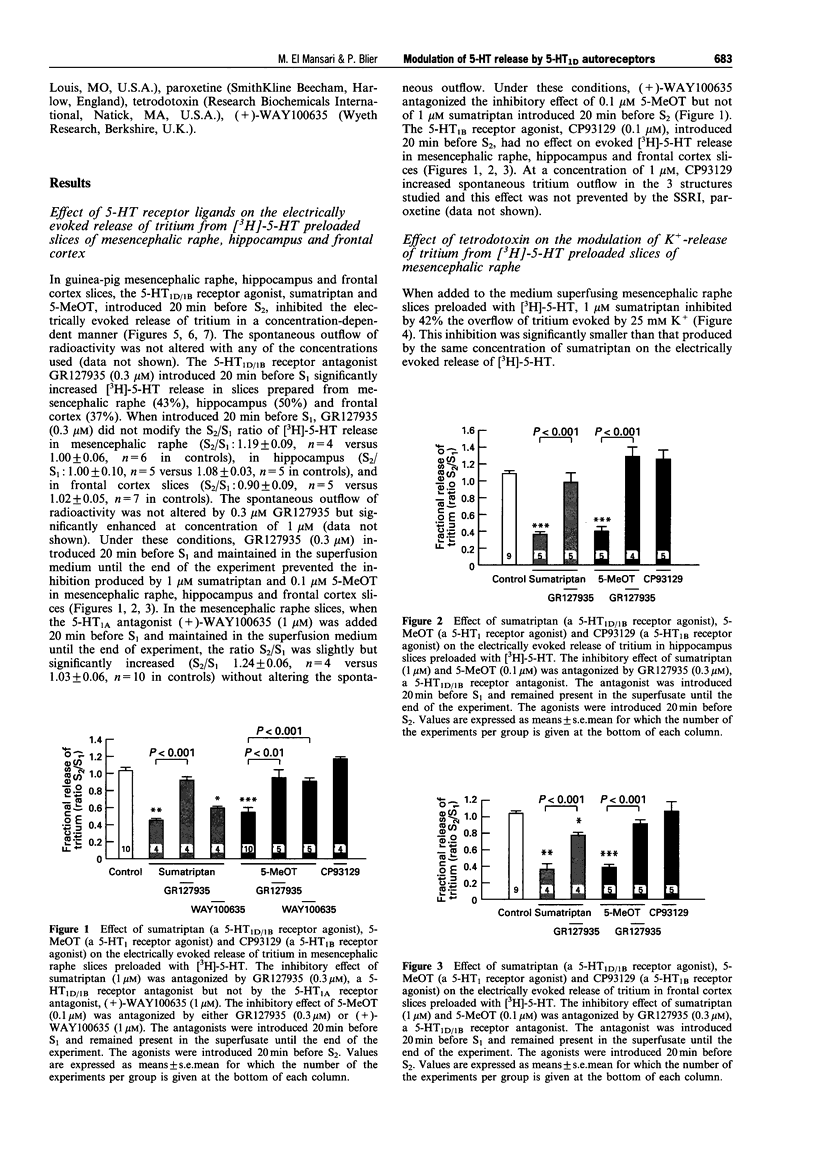
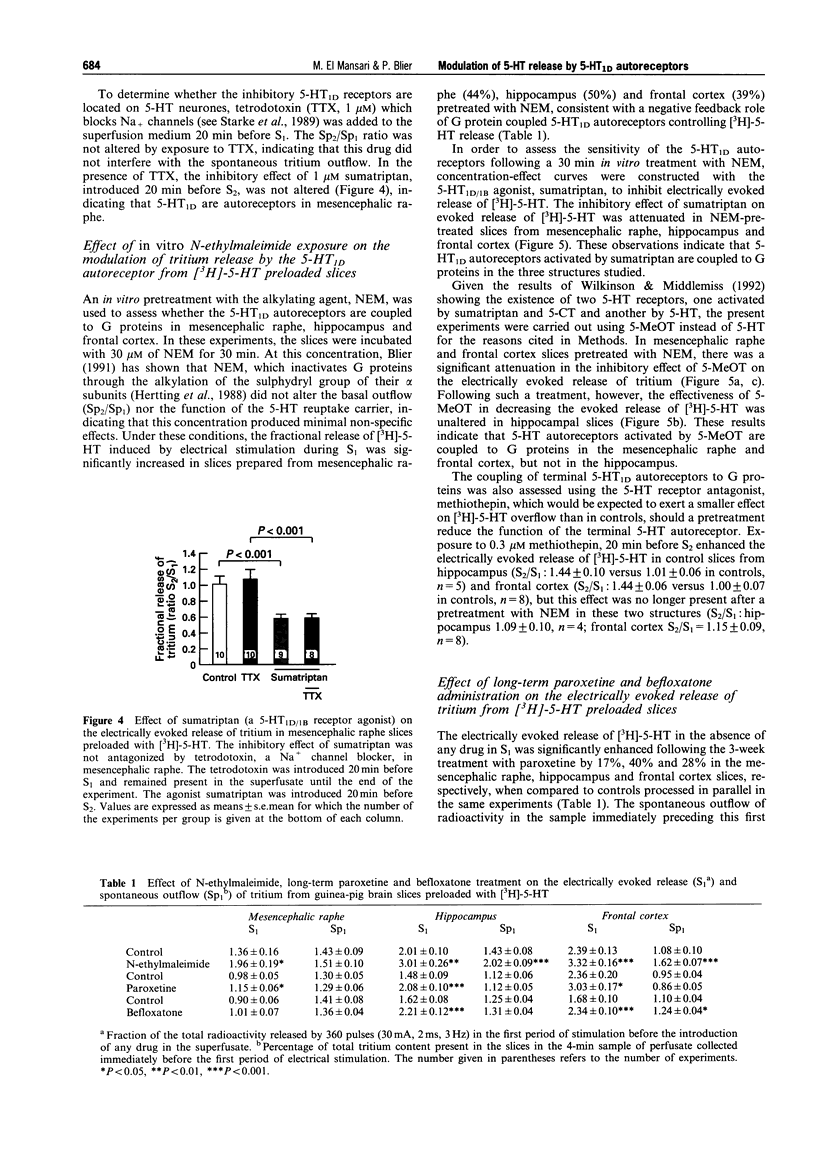
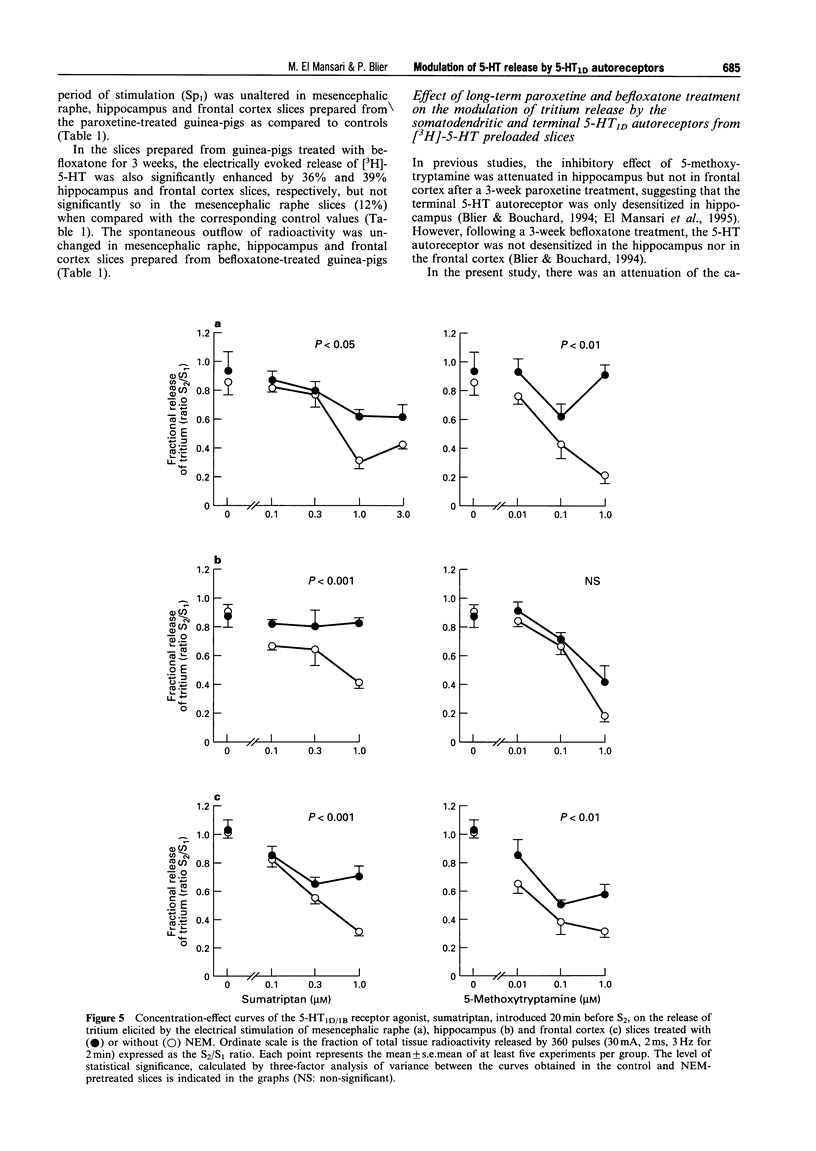
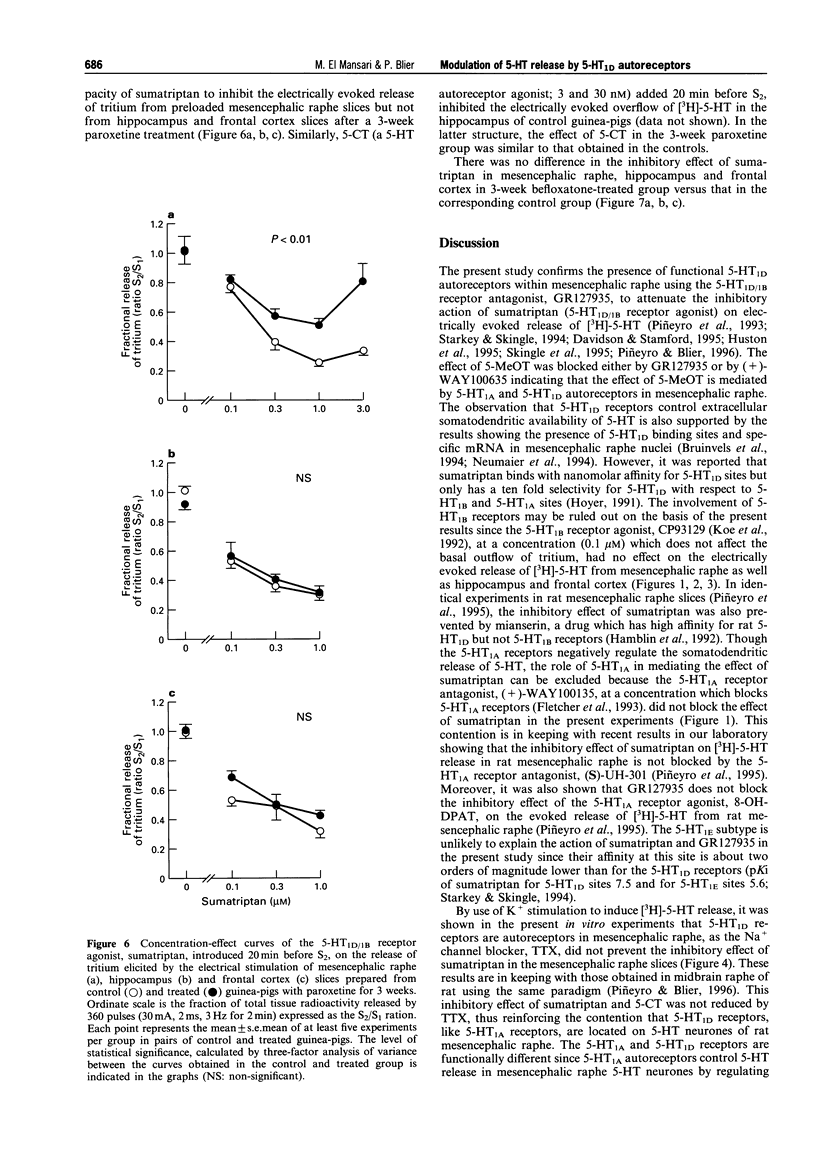
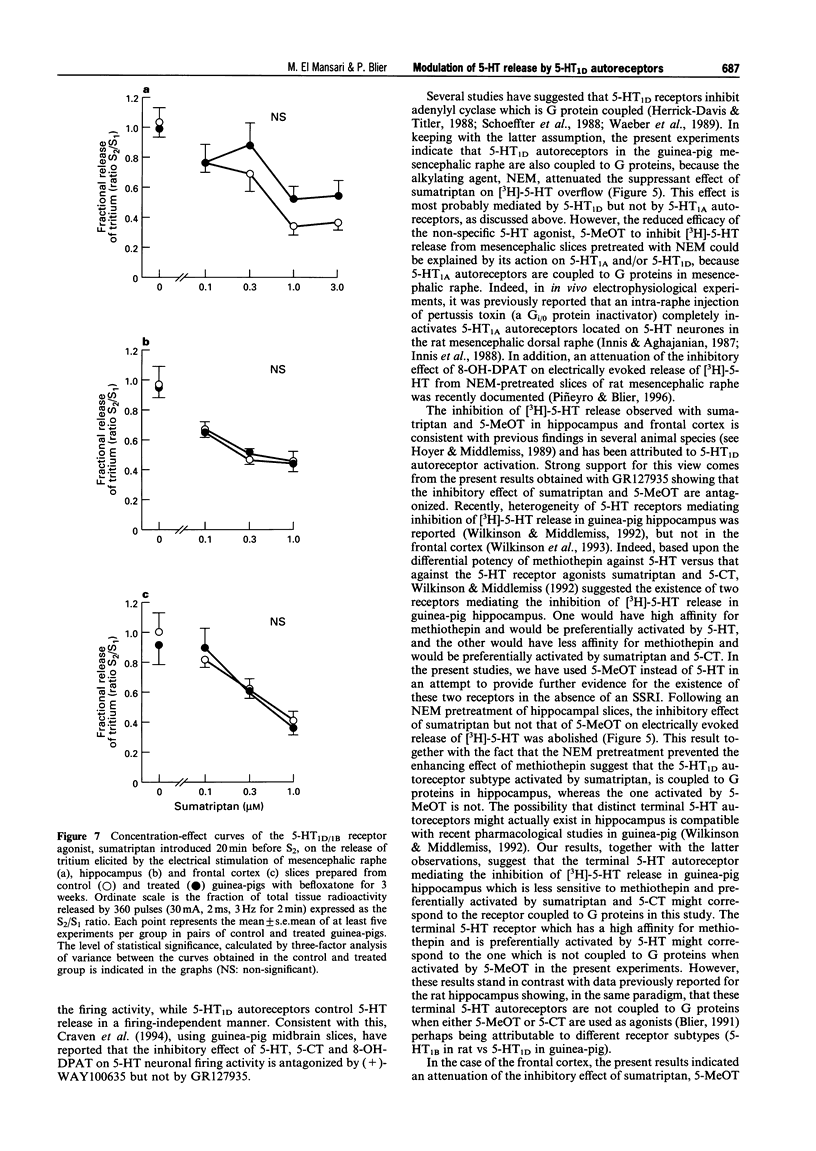
1. The aims of the present study were (i) to characterize further the pharmacology of 5-HT1D autoreceptors modulating 5-HT release in guinea-pig mesencephalic raphe, hippocampus and frontal cortex; (ii) to determine whether 5-HT1D receptors in the mesencephalic raphe are located on 5-HT neurones; (iii) to determine whether 5-HT1D autoreceptors are coupled to G proteins; and (iv) to assess their sensitivity following long-term 5-HT reuptake blockade and inhibition of type-A monoamine oxidase. 2. In mesencephalic raphe, hippocampus and frontal cortex slices, the 5-HT1D/1B receptor agonist, sumatriptan and the 5-HT1 receptor agonist, 5-methoxytryptamine (5-MeOT) but not the 5-HT1B receptor agonist, CP93129, inhibited electrically the evoked release of [3H]-5-HT in a concentration-dependent manner. This effect was antagonized by the 5-HT1D/1B receptor antagonist GR127935 in the three structures, but not by the 5-HT1A receptor antagonist, (+)-WAY100635 in mesencephalic raphe slices. These results confirm the presence of functional 5-HT1D autoreceptors controlling 5-HT release within the mesencephalic raphe as well as in terminal regions. 3. The inhibitory effect of sumatriptan on K(+)-evoked release of [3H]-5-HT was not reduced by the addition of the Na+ channel blocker, tetrodotoxin to the superfusion medium, suggesting that these 5-HT1D receptors in the mesencephalic raphe are located on 5-HT neurones and may be considered autoreceptors. 4. The in vitro treatment with the alkylating agent N-ethylmaleimide (NEM) was used to determine whether these 5-HT1D autoreceptors are coupled to G proteins. The inhibitory effect of sumatriptan on electrically evoked release of [3H]-5-HT was attenuated in NEM-pretreated slices from mesencephalic raphe, hippocampus and frontal cortex, indicating that the 5-HT1D autoreceptors activated by sumatriptan are coupled to G proteins in these three structures. Taken together with our previous results, this suggests that, in addition to the 5-HT1D autoreceptor activated by sumatriptan, another subtype of 5-HT autoreceptor is activated by 5-MeOT in the hippocampus. 5. Following a 3-week treatment with the selective 5-HT reuptake inhibitor, paroxetine (10 mg kg-1 day-1) and a 48 h washout period, the electrically evoked release of [3H]-5-HT was enhanced in mesencephalic raphe, hippocampus and frontal cortex slices. There was an attenuation of the capacity of sumatriptan to inhibit the evoked release of [3H]-5-HT from mesencephalic raphe slices but not from frontal cortex and hippocampus slices. Only in the latter structure was the suppressant effect of 5-MeOT attenuated. After a 3-week treatment with the reversible type-A monoamine oxidase inhibitor, befloxatone (0.75 mg kg-1 day-1) and 48 h washout period, the effectiveness of sumatriptan and 5-MeOT on the evoked release of [3H]-5-HT was unaltered in the same brain structures. 6. The enhancement of [3H]-5-HT release by long-term paroxetine treatment is possibly due to a desensitization of 5-HT1D autoreceptors activated by sumatriptan in mesencephalic raphe and by terminal 5-HT autoreceptors activated by 5-MeOT in hippocampus. In the case of the frontal cortex, it appears that 5-MeOT and sumatriptan may act on the same 5-HT1D autoreceptor which is not desensitized either after paroxetine or befloxatone treatment, as previously reported.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adell A., Artigas F. Differential effects of clomipramine given locally or systemically on extracellular 5-hydroxytryptamine in raphe nuclei and frontal cortex. An in vivo brain microdialysis study. Naunyn Schmiedebergs Arch Pharmacol. 1991 Mar;343(3):237–244. doi: 10.1007/BF00251121. [DOI] [PubMed] [Google Scholar]
- Baumann P. A., Waldmeier P. C. Further evidence for negative feedback control of serotonin release in the central nervous system. Naunyn Schmiedebergs Arch Pharmacol. 1981 Aug;317(1):36–43. doi: 10.1007/BF00506254. [DOI] [PubMed] [Google Scholar]
- Blier P., Bouchard C. Functional characterization of a 5-HT3 receptor which modulates the release of 5-HT in the guinea-pig brain. Br J Pharmacol. 1993 Jan;108(1):13–22. doi: 10.1111/j.1476-5381.1993.tb13433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P., Bouchard C. Modulation of 5-HT release in the guinea-pig brain following long-term administration of antidepressant drugs. Br J Pharmacol. 1994 Oct;113(2):485–495. doi: 10.1111/j.1476-5381.1994.tb17015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P., Monroe P. J., Bouchard C., Smith D. L., Smith D. J. 5-HT3 receptors which modulate [3H]5-HT release in the guinea pig hypothalamus are not autoreceptors. Synapse. 1993 Oct;15(2):143–148. doi: 10.1002/syn.890150206. [DOI] [PubMed] [Google Scholar]
- Blier P. Terminal serotonin autoreceptor function in the rat hippocampus is not modified by pertussis and cholera toxins. Naunyn Schmiedebergs Arch Pharmacol. 1991 Aug;344(2):160–166. doi: 10.1007/BF00167213. [DOI] [PubMed] [Google Scholar]
- Bruinvels A. T., Landwehrmeyer B., Gustafson E. L., Durkin M. M., Mengod G., Branchek T. A., Hoyer D., Palacios J. M. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994 Mar-Apr;33(3-4):367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Davidson C., Stamford J. A. Evidence that 5-hydroxytryptamine release in rat dorsal raphé nucleus is controlled by 5-HT1A, 5-HT1B and 5-HT1D autoreceptors. Br J Pharmacol. 1995 Mar;114(6):1107–1109. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A., Bill D. J., Bill S. J., Cliffe I. A., Dover G. M., Forster E. A., Haskins J. T., Jones D., Mansell H. L., Reilly Y. WAY100135: a novel, selective antagonist at presynaptic and postsynaptic 5-HT1A receptors. Eur J Pharmacol. 1993 Jun 24;237(2-3):283–291. doi: 10.1016/0014-2999(93)90280-u. [DOI] [PubMed] [Google Scholar]
- Galzin A. M., Moret C., Verzier B., Langer S. Z. Interaction between tricyclic and nontricyclic 5-hydroxytryptamine uptake inhibitors and the presynaptic 5-hydroxytryptamine inhibitory autoreceptors in the rat hypothalamus. J Pharmacol Exp Ther. 1985 Oct;235(1):200–211. [PubMed] [Google Scholar]
- Galzin A. M., Poirier M. F., Lista A., Chodkiewicz J. P., Blier P., Ramdine R., Loô H., Roux F. X., Redondo A., Langer S. Z. Characterization of the 5-hydroxytryptamine receptor modulating the release of 5-[3H]hydroxytryptamine in slices of the human neocortex. J Neurochem. 1992 Oct;59(4):1293–1301. doi: 10.1111/j.1471-4159.1992.tb08440.x. [DOI] [PubMed] [Google Scholar]
- Hamblin M. W., Metcalf M. A. Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor. Mol Pharmacol. 1991 Aug;40(2):143–148. [PubMed] [Google Scholar]
- Herrick-Davis K., Titeler M., Leonhardt S., Struble R., Price D. Serotonin 5-HT1D receptors in human prefrontal cortex and caudate: interaction with a GTP binding protein. J Neurochem. 1988 Dec;51(6):1906–1912. doi: 10.1111/j.1471-4159.1988.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Middlemiss D. N. Species differences in the pharmacology of terminal 5-HT autoreceptors in mammalian brain. Trends Pharmacol Sci. 1989 Apr;10(4):130–132. doi: 10.1016/0165-6147(89)90159-4. [DOI] [PubMed] [Google Scholar]
- Hutson P. H., Bristow L. J., Cunningham J. R., Hogg J. E., Longmore J., Murray F., Pearce D., Razzaque Z., Saywell K., Tricklebank M. D. The effects of GR127935, a putative 5-HT1D receptor antagonist, on brain 5-HT metabolism, extracellular 5-HT concentration and behaviour in the guinea pig. Neuropharmacology. 1995 Apr;34(4):383–392. doi: 10.1016/0028-3908(95)00016-y. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Aghajanian G. K. Pertussis toxin blocks autoreceptor-mediated inhibition of dopaminergic neurons in rat substantia nigra. Brain Res. 1987 May 12;411(1):139–143. doi: 10.1016/0006-8993(87)90690-1. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Nestler E. J., Aghajanian G. K. Evidence for G protein mediation of serotonin- and GABAB-induced hyperpolarization of rat dorsal raphe neurons. Brain Res. 1988 Aug 30;459(1):27–36. doi: 10.1016/0006-8993(88)90282-x. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L. Single unit activity of brain monoamine-containing neurons in freely moving animals. Ann N Y Acad Sci. 1986;473:70–77. doi: 10.1111/j.1749-6632.1986.tb23604.x. [DOI] [PubMed] [Google Scholar]
- Limberger N., Deicher R., Starke K. Species differences in presynaptic serotonin autoreceptors: mainly 5-HT1B but possibly in addition 5-HT1D in the rat, 5-HT1D in the rabbit and guinea-pig brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1991 Apr;343(4):353–364. doi: 10.1007/BF00179039. [DOI] [PubMed] [Google Scholar]
- Maura G., Thellung S., Andrioli G. C., Ruelle A., Raiteri M. Release-regulating serotonin 5-HT1D autoreceptors in human cerebral cortex. J Neurochem. 1993 Mar;60(3):1179–1182. doi: 10.1111/j.1471-4159.1993.tb03274.x. [DOI] [PubMed] [Google Scholar]
- Middlemiss D. N. The putative 5-HT1 receptor agonist, RU 24969, inhibits the efflux of 5-hydroxytryptamine from rat frontal cortex slices by stimulation of the 5-HT autoreceptor. J Pharm Pharmacol. 1985 Jun;37(6):434–437. doi: 10.1111/j.2042-7158.1985.tb03032.x. [DOI] [PubMed] [Google Scholar]
- Mongeau R., de Montigny C., Blier P. Electrophysiologic evidence for desensitization of alpha 2-adrenoceptors on serotonin terminals following long-term treatment with drugs increasing norepinephrine synaptic concentration. Neuropsychopharmacology. 1994 Feb;10(1):41–51. doi: 10.1038/npp.1994.6. [DOI] [PubMed] [Google Scholar]
- Mundey M. K., Fletcher A., Marsden C. A. Effect of the putative 5-HT1A antagonists WAY100135 and SDZ 216-525 on 5-HT neuronal firing in the guinea-pig dorsal raphe nucleus. Neuropharmacology. 1994 Jan;33(1):61–66. doi: 10.1016/0028-3908(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Piñeyro G., Blier P. Regulation of 5-hydroxytryptamine release from rat midbrain raphe nuclei by 5-hydroxytryptamine1D receptors: effect of tetrodotoxin, G protein inactivation and long-term antidepressant administration. J Pharmacol Exp Ther. 1996 Feb;276(2):697–707. [PubMed] [Google Scholar]
- Piñeyro G., de Montigny C., Blier P. 5-HT1D receptors regulate 5-HT release in the rat raphe nuclei. In vivo voltammetry and in vitro superfusion studies. Neuropsychopharmacology. 1995 Nov;13(3):249–260. doi: 10.1016/0893-133X(95)00109-Q. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Waeber C., Palacios J. M., Hoyer D. The 5-hydroxytryptamine 5-HT1D receptor subtype is negatively coupled to adenylate cyclase in calf substantia nigra. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):602–608. doi: 10.1007/BF00175784. [DOI] [PubMed] [Google Scholar]
- Skingle M., Sleight A. J., Feniuk W. Effects of the 5-HT1D receptor antagonist GR127935 on extracellular levels of 5-HT in the guinea-pig frontal cortex as measured by microdialysis. Neuropharmacology. 1995 Apr;34(4):377–382. doi: 10.1016/0028-3908(94)00167-q. [DOI] [PubMed] [Google Scholar]
- Starke K., Göthert M., Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989 Jul;69(3):864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Starkey S. J., Skingle M. 5-HT1D as well as 5-HT1A autoreceptors modulate 5-HT release in the guinea-pig dorsal raphé nucleus. Neuropharmacology. 1994 Mar-Apr;33(3-4):393–402. doi: 10.1016/0028-3908(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Waeber C., Schoeffter P., Palacios J. M., Hoyer D. 5-HT1D receptors in guinea-pig and pigeon brain. Radioligand binding and biochemical studies. Naunyn Schmiedebergs Arch Pharmacol. 1989 Nov;340(5):479–485. doi: 10.1007/BF00260601. [DOI] [PubMed] [Google Scholar]
- Weinshank R. L., Zgombick J. M., Macchi M. J., Branchek T. A., Hartig P. R. Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1D alpha and 5-HT1D beta. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3630–3634. doi: 10.1073/pnas.89.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson L. O., Hawkins L. M., Beer M. S., Hibert M. F., Middlemiss D. N. Stereoselective actions of the isomers of metitepine at 5-HT1D receptors in the guinea pig brain. Neuropharmacology. 1993 Mar;32(3):205–208. doi: 10.1016/0028-3908(93)90101-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson L. O., Middlemiss D. N. Metitepine distinguishes two receptors mediating inhibition of [3H]-5-hydroxytryptamine release in guinea pig hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1992 Jun;345(6):696–699. doi: 10.1007/BF00164585. [DOI] [PubMed] [Google Scholar]
- Zgombick J. M., Borden L. A., Cochran T. L., Kucharewicz S. A., Weinshank R. L., Branchek T. A. Dual coupling of cloned human 5-hydroxytryptamine1D alpha and 5-hydroxytryptamine1D beta receptors stably expressed in murine fibroblasts: inhibition of adenylate cyclase and elevation of intracellular calcium concentrations via pertussis toxin-sensitive G protein(s). Mol Pharmacol. 1993 Sep;44(3):575–582. [PubMed] [Google Scholar]
- el Mansari M., Bouchard C., Blier P. Alteration of serotonin release in the guinea pig orbito-frontal cortex by selective serotonin reuptake inhibitors. Relevance to treatment of obsessive-compulsive disorder. Neuropsychopharmacology. 1995 Oct;13(2):117–127. doi: 10.1016/0893-133X(95)00045-F. [DOI] [PubMed] [Google Scholar]


