Abstract
1. The effects of norbormide on the contractility of endothelium-deprived rat, guinea-pig, mouse, and human artery rings, and of freshly isolated smooth muscle cells of rat caudal artery were investigated. In addition, the effect of norbormide on intracellular calcium levels of A7r5 cells was evaluated. 2. In resting rat mesenteric, renal, and caudal arteries, norbormide (0.5-50 microM) induced a concentration-dependent contractile effect. In rat caudal artery, the contraction was very slowly reversible on washing, completely abolished in the absence of extracellular calcium, and antagonized by high concentrations (10-800 microM) of verapamil. The norbormide effect persisted upon removal of either extracellular Na+ or K+. The contractile effect of norbormide was observed also in single, freshly isolated smooth muscle cells from rat caudal artery. 3. In resting rat and guinea-pig aortae, guinea-pig mesenteric artery, mouse caudal artery, and human subcutaneous resistance arteries, norbormide did not induce contraction. When these vessels were contracted by 80 mM KCl, norbormide (10-100 microM) caused relaxation. Norbormide inhibited the response to Ca2+ of rat aorta incubated in 80 mM KCl/Ca2(+)-free medium. Norbormide (up to 100 microM) was ineffective in phenylephrine-contracted guinea-pig and rat aorta. 4. In A7r5 cells, a cell line from rat aorta, norbormide prevented high K(+)- but not 5-hydroxytryptamine-induced intracellular calcium transients. 5. These findings indicate that in vitro, norbormide induces a myogenic contraction, selective for the rat small vessels, by promoting calcium entry in smooth muscle cells, presumably through calcium channels. In rat aorta and arteries from other mammals, norbormide behaves like a calcium channel entry blocker.
Full text
PDF

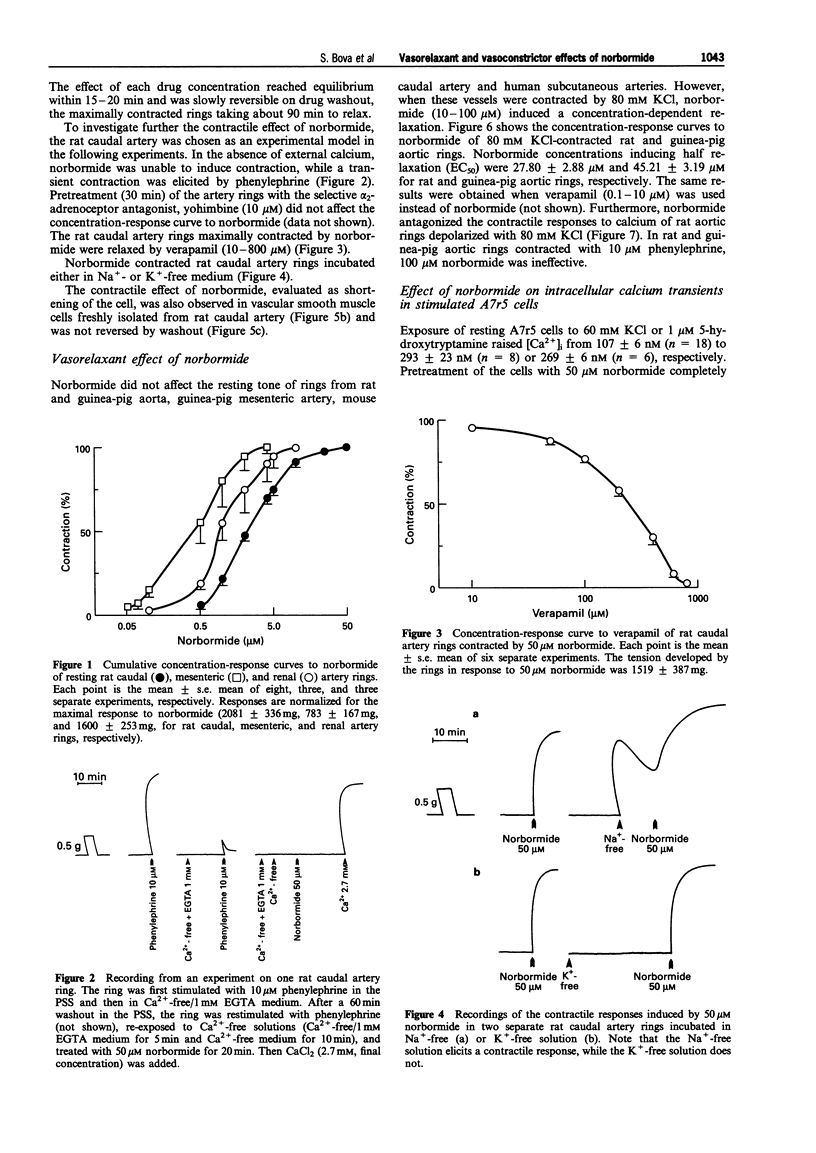
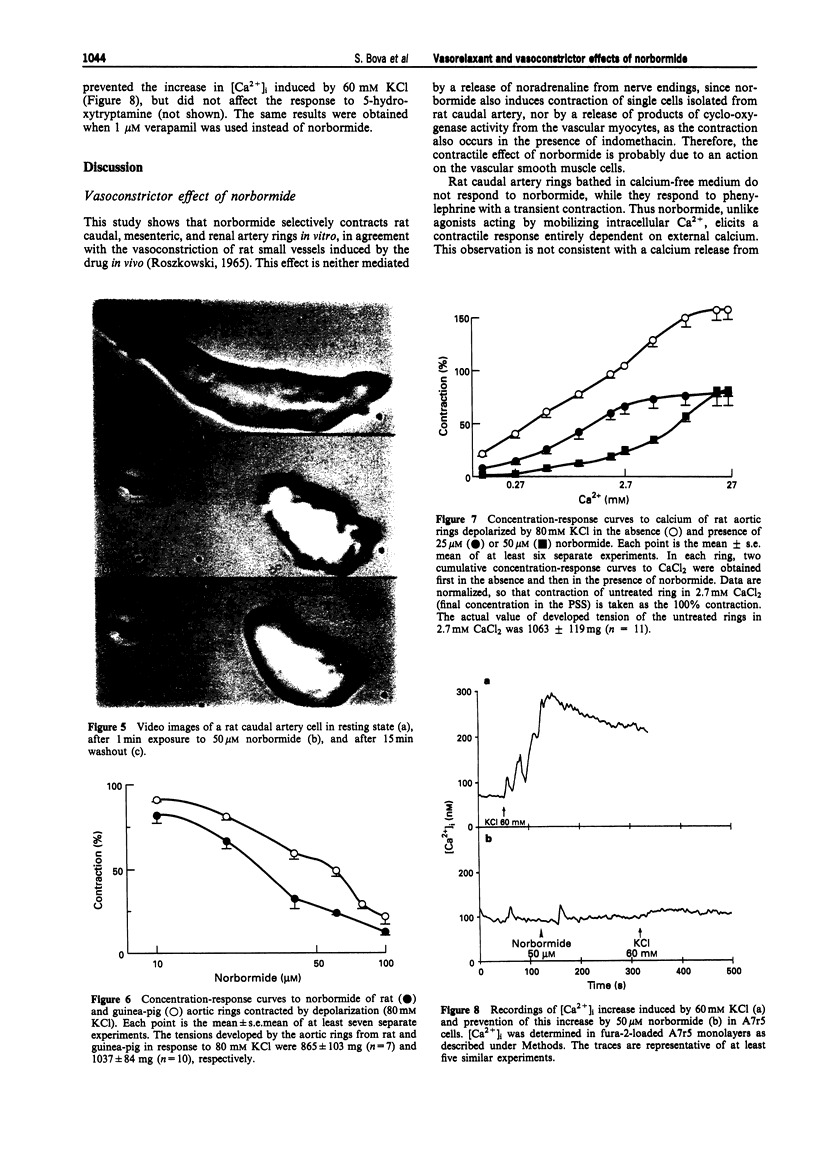


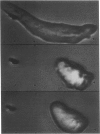
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Hidaka H. Vasodilatory action of HA1004 [N-(2-guanidinoethyl)-5-isoquinolinesulfonamide], a novel calcium antagonist with no effect on cardiac function. J Pharmacol Exp Ther. 1984 Oct;231(1):141–145. [PubMed] [Google Scholar]
- Ashida T., Blaustein M. P. Regulation of cell calcium and contractility in mammalian arterial smooth muscle: the role of sodium-calcium exchange. J Physiol. 1987 Nov;392:617–635. doi: 10.1113/jphysiol.1987.sp016800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova S., Cargnelli G., Luciani S. Na/Ca exchange and tension development in vascular smooth muscle: effect of amiloride. Br J Pharmacol. 1988 Mar;93(3):601–608. doi: 10.1111/j.1476-5381.1988.tb10316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova S., Rossi G., Luciani S., Debetto P., Pessina A. C., Cargnelli G. Effect of subthreshold ouabain on the tone of guinea-pig aortic strips following repeated noradrenaline stimulation. Br J Pharmacol. 1994 Apr;111(4):1067–1072. doi: 10.1111/j.1476-5381.1994.tb14853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauvin C., Loutzenhiser R., Van Breemen C. Mechanisms of calcium antagonist-induced vasodilation. Annu Rev Pharmacol Toxicol. 1983;23:373–396. doi: 10.1146/annurev.pa.23.040183.002105. [DOI] [PubMed] [Google Scholar]
- Cauvin C., Saida K., van Breemen C. Extracellular Ca2+ dependence and diltiazem inhibition of contraction in rabbit conduit arteries and mesenteric resistance vessels. Blood Vessels. 1984;21(1):23–31. doi: 10.1159/000158491. [DOI] [PubMed] [Google Scholar]
- Furspan P. B. WAY 120,491 activates ATP-sensitive potassium channels in rat tail artery. Eur J Pharmacol. 1992 Nov 17;223(2-3):201–203. doi: 10.1016/0014-2999(92)94841-i. [DOI] [PubMed] [Google Scholar]
- Goldman W. F., Bova S., Blaustein M. P. Measurement of intracellular Ca2+ in cultured arterial smooth muscle cells using Fura-2 and digital imaging microscopy. Cell Calcium. 1990 Feb-Mar;11(2-3):221–231. doi: 10.1016/0143-4160(90)90073-4. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Jim K. F., Matthews W. D. Role of extracellular calcium in contractions produced by activation of postsynaptic alpha-2 adrenoceptors in the canine saphenous vein. J Pharmacol Exp Ther. 1985 Jul;234(1):161–165. [PubMed] [Google Scholar]
- Jones A. W., Bylund D. B., Forte L. R. cAMP-dependent reduction in membrane fluxes during relaxation of arterial smooth muscle. Am J Physiol. 1984 Feb;246(2 Pt 2):H306–H311. doi: 10.1152/ajpheart.1984.246.2.H306. [DOI] [PubMed] [Google Scholar]
- Karaki H., Ozaki H., Urakawa N. Effects of ouabain and potassium-free solution on the contraction of isolated blood vessels. Eur J Pharmacol. 1978 Apr 15;48(4):439–443. doi: 10.1016/0014-2999(78)90172-3. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M. Effects of nitroprusside and 8-bromo-cyclic GMP on the contractile activity of the rat aorta. J Pharmacol Exp Ther. 1983 Jan;224(1):100–107. [PubMed] [Google Scholar]
- Nabel E. G., Berk B. C., Brock T. A., Smith T. W. Na+-Ca2+ exchange in cultured vascular smooth muscle cells. Circ Res. 1988 Mar;62(3):486–493. doi: 10.1161/01.res.62.3.486. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Karaki H., Urakawa N. Possible role of Na-Ca exchange mechanism in the contractions induced in guinea-pig aorta by potassium free solution and ouabain. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;304(3):203–209. doi: 10.1007/BF00507959. [DOI] [PubMed] [Google Scholar]
- Poos G. I., Mohrbacher R. J., Carson E. L., Paragamian V., Puma B. M., Rasmussen C. R., Roszkowski A. P. Structure-activity studies with the selective rat toxicant norbormide. J Med Chem. 1966 Jul;9(4):537–540. doi: 10.1021/jm00322a021. [DOI] [PubMed] [Google Scholar]
- ROSZKOWSKI A. P., POOS G. I., MOHRBACHER R. J. SELECTIVE RAT TOXICANT. Science. 1964 Apr 24;144(3617):412–413. doi: 10.1126/science.144.3617.412. [DOI] [PubMed] [Google Scholar]
- Roszkowski A. P. The pharmacological properties of norbormide, a selective rat toxicant. J Pharmacol Exp Ther. 1965 Aug;149(2):288–299. [PubMed] [Google Scholar]
- Schultz K. D., Böhme E., Kreye V. A., Schultz G. Relaxation of hormonally stimulated smooth muscular tissues by the 8-bromo derivative of cyclic GMP. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jan;306(1):1–9. doi: 10.1007/BF00515586. [DOI] [PubMed] [Google Scholar]
- Shinjoh M., Nakaki T., Otsuka Y., Sasakawa N., Kato R. Vascular smooth muscle contraction induced by Na+ channel activators, veratridine and batrachotoxin. Eur J Pharmacol. 1991 Nov 26;205(2):199–202. doi: 10.1016/0014-2999(91)90820-g. [DOI] [PubMed] [Google Scholar]
- Woolfson R. G., Hilton P. J., Poston L. Effects of ouabain and low sodium on contractility of human resistance arteries. Hypertension. 1990 Jun;15(6 Pt 1):583–590. doi: 10.1161/01.hyp.15.6.583. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]