Abstract
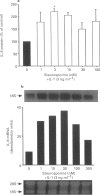
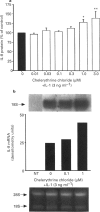
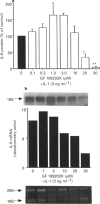
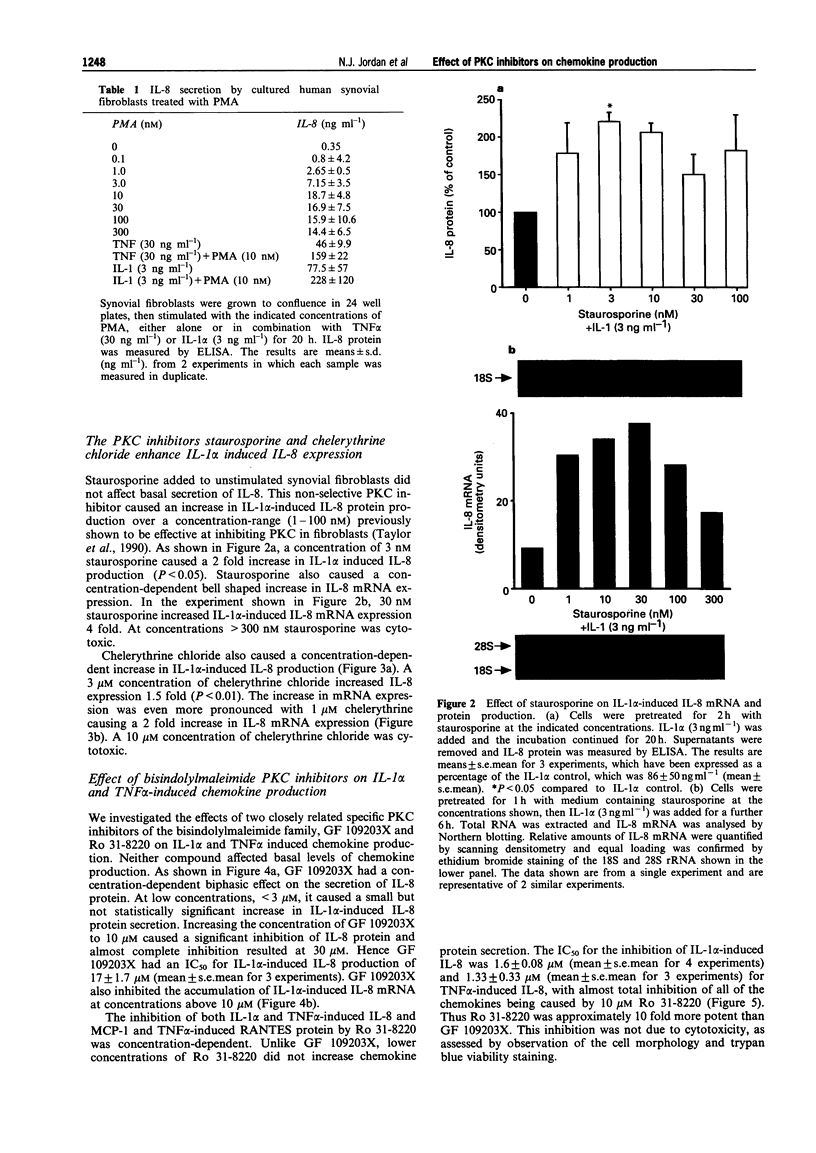
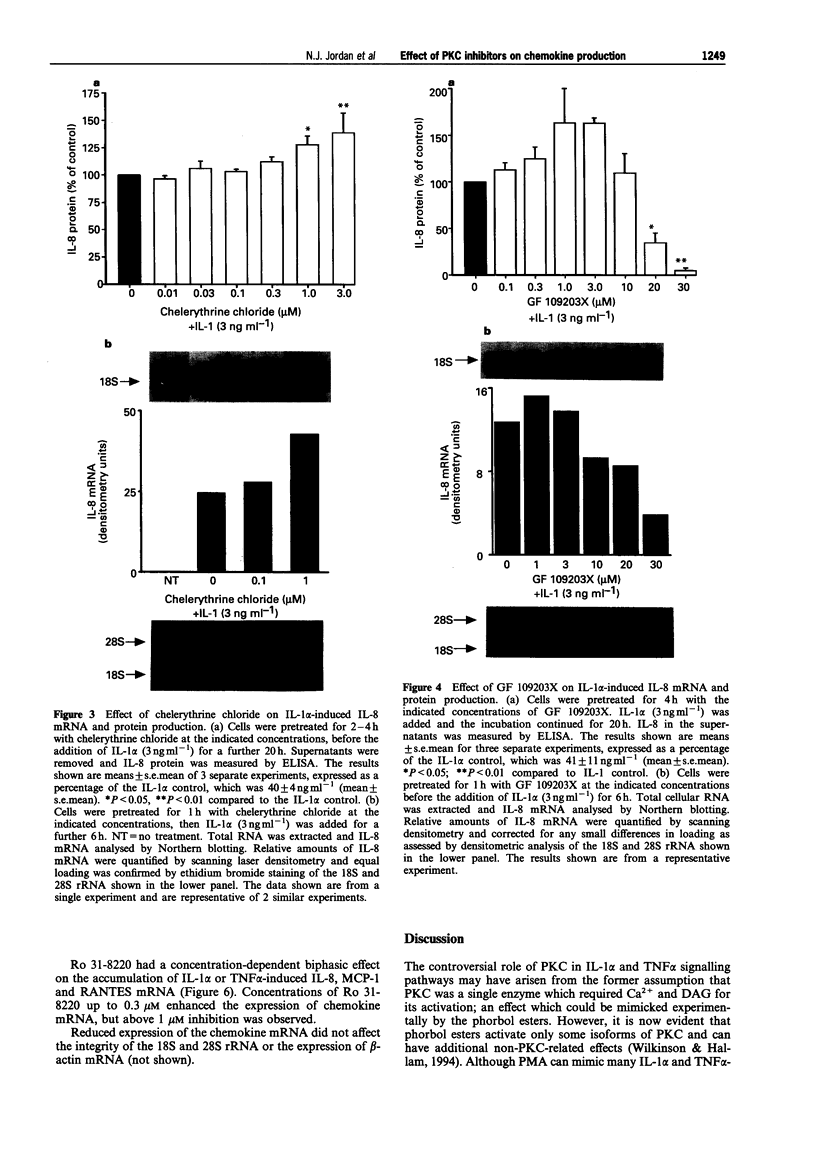
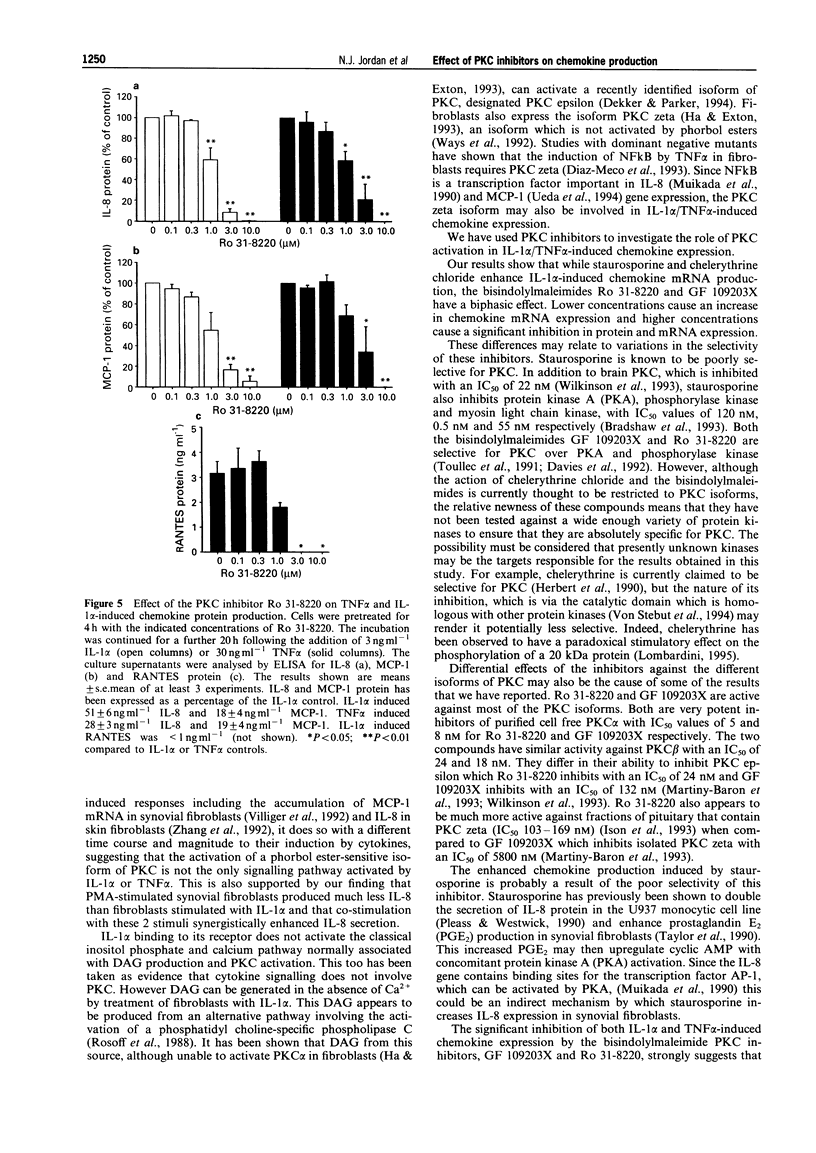
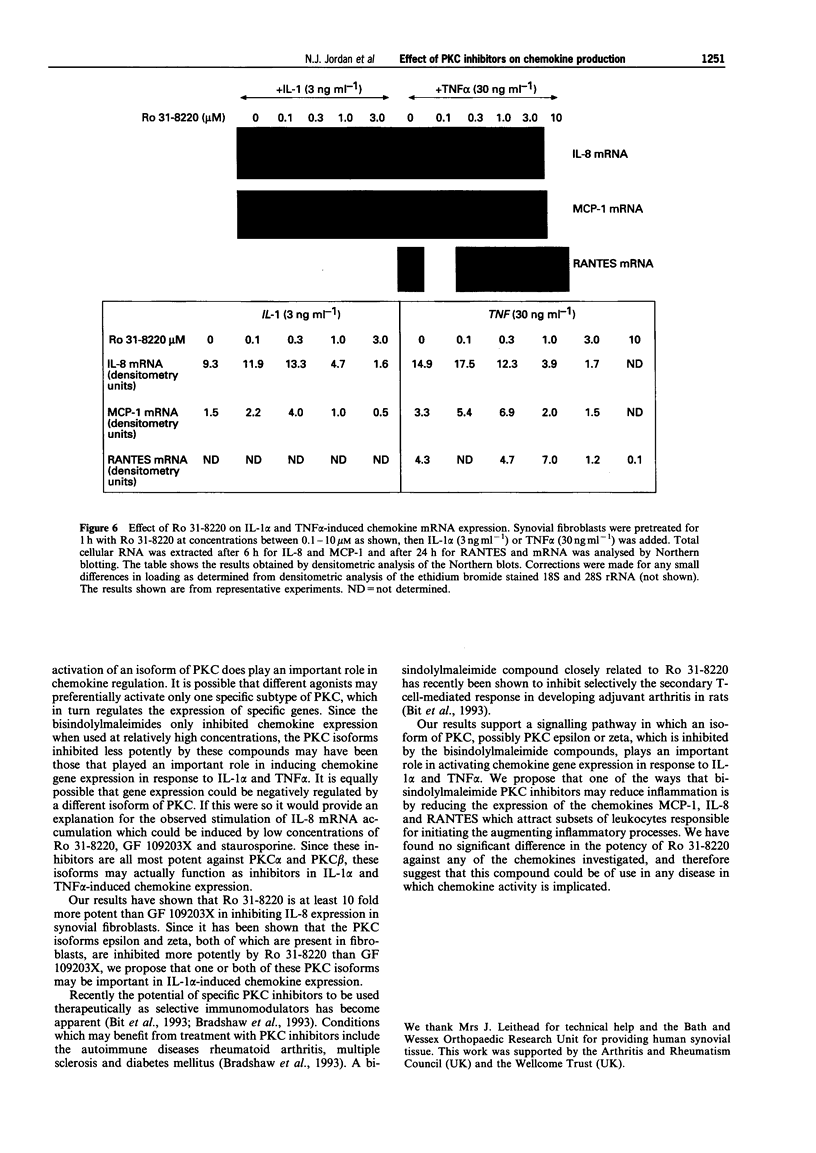
1. Rheumatoid arthritis is associated with the accumulation and activation of selected populations of inflammatory cells within the arthritic joint. One putative signal for this process is the production, by resident cells, of a group of inflammatory mediators known as the chemokines. 2. The chemokines interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1) and RANTES (regulated on activation normal T-cell expressed and presumably secreted) are target-cell specific chemoattractants produced by synovial fibroblasts in response to stimulation with interleukin-1 alpha (IL-1 alpha) or tumour necrosis factor alpha (TNF alpha). The signalling pathways involved in their production are not well defined. We therefore used four different protein kinase C inhibitors to investigate the role of this kinase in the regulation of chemokine mRNA and protein expression in human cultured synovial fibroblasts. 3. The non-selective PKC inhibitor, staurosporine (1-300 nM) significantly increased the production of IL-1 alpha-induced IL-8 mRNA and protein. A specific PKC inhibitor, chelerythrine chloride (0.1-3 microM), also caused a small concentration-dependent increase in IL-8 mRNA and protein production. In contrast, 3-[1-[3-(amidinothio)propyl]-3-indoly]-4-(1-methyl-3-indolyl )- 1H-pyrrole-2,5-dione methanesulphonate (Ro 31-8220) and 2[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3- yl)-maleimide (GF 109203X), two selective PKC inhibitors of the substituted bisindolylmaleimide family had a concentration-dependent biphasic effect on IL-1 alpha or TNF alpha-induced chemokine expression. At low concentrations they caused a stimulation in chemokine production, which was especially evident at the mRNA level. At higher concentrations both inhibited IL-1 alpha or TNF alpha-induced chemokine mRNA and protein production. Ro 31-8220 was 10 fold more potent than GF 109203X, with an IC50 of 1.6 +/- 0.08 microM (mean +/- s.e.mean, n = 4) for IL-1 alpha induced IL-8 production. Ro 31-8220 also inhibited the expression of IL-1 alpha or TNF alpha-induced MCP-1 and RANTES mRNA with a similar potency. 4. The stimulatory effect of staurosporine is discussed in relation to the known poor selectivity of this inhibitor for PKC. It is proposed that activation of an isoform of PKC, possibly PKC epsilon or zeta, which is inhibited by higher concentrations of the bisinodolylmaleimides, plays a role in the regulation of chemokine expression induced by IL-1 alpha or TNF alpha in synovial cells. 5. The inhibition of chemokine production by bisindolylmaleimide compounds heralds a novel approach for future anti-inflammatory therapies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bit R. A., Davis P. D., Elliott L. H., Harris W., Hill C. H., Keech E., Kumar H., Lawton G., Maw A., Nixon J. S. Inhibitors of protein kinase C. 3. Potent and highly selective bisindolylmaleimides by conformational restriction. J Med Chem. 1993 Jan 8;36(1):21–29. doi: 10.1021/jm00053a003. [DOI] [PubMed] [Google Scholar]
- Bradshaw D., Hill C. H., Nixon J. S., Wilkinson S. E. Therapeutic potential of protein kinase C inhibitors. Agents Actions. 1993 Jan;38(1-2):135–147. doi: 10.1007/BF02027225. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Zachariae C. O., Chantry D., Larsen C. G., Turner M., Maini R. N., Matsushima K., Feldmann M. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990 Sep;20(9):2141–2144. doi: 10.1002/eji.1830200938. [DOI] [PubMed] [Google Scholar]
- Brown Z., Strieter R. M., Chensue S. W., Ceska M., Lindley I., Neild G. H., Kunkel S. L., Westwick J. Cytokine-activated human mesangial cells generate the neutrophil chemoattractant, interleukin 8. Kidney Int. 1991 Jul;40(1):86–90. doi: 10.1038/ki.1991.184. [DOI] [PubMed] [Google Scholar]
- Bédard P. A., Golds E. E. Cytokine-induced expression of mRNAs for chemotactic factors in human synovial cells and fibroblasts. J Cell Physiol. 1993 Feb;154(2):433–441. doi: 10.1002/jcp.1041540227. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis P. D., Elliott L. H., Harris W., Hill C. H., Hurst S. A., Keech E., Kumar M. K., Lawton G., Nixon J. S., Wilkinson S. E. Inhibitors of protein kinase C. 2. Substituted bisindolylmaleimides with improved potency and selectivity. J Med Chem. 1992 Mar 20;35(6):994–1001. doi: 10.1021/jm00084a004. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco D., Kunkel S. L., Strieter R. M., Basha M., Zurier R. B. Interleukin-1 induced gene expression of neutrophil activating protein (interleukin-8) and monocyte chemotactic peptide in human synovial cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):411–416. doi: 10.1016/0006-291x(91)91431-b. [DOI] [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J. Protein kinase C--a question of specificity. Trends Biochem Sci. 1994 Feb;19(2):73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Berra E., Municio M. M., Sanz L., Lozano J., Dominguez I., Diaz-Golpe V., Lain de Lera M. T., Alcamí J., Payá C. V. A dominant negative protein kinase C zeta subspecies blocks NF-kappa B activation. Mol Cell Biol. 1993 Aug;13(8):4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy G. R., Chua S. P., Wong N. S., Ng S. B., Tan Y. H. Interleukin 1 and tumor necrosis factor activate common multiple protein kinases in human fibroblasts. J Biol Chem. 1991 Aug 5;266(22):14343–14352. [PubMed] [Google Scholar]
- Ha K. S., Exton J. H. Differential translocation of protein kinase C isozymes by thrombin and platelet-derived growth factor. A possible function for phosphatidylcholine-derived diacylglycerol. J Biol Chem. 1993 May 15;268(14):10534–10539. [PubMed] [Google Scholar]
- Herbert J. M., Augereau J. M., Gleye J., Maffrand J. P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990 Nov 15;172(3):993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Ison A. J., Johnson M. S., MacEwan D. J., Simpson J., Clegg R. A., Connor K., Mitchell R. Biochemical characterisation of an apparently novel isoform of protein kinase C in pituitary. Biochem Soc Trans. 1993 Nov;21(4):386S–386S. doi: 10.1042/bst021386s. [DOI] [PubMed] [Google Scholar]
- Johannes F. J., Prestle J., Eis S., Oberhagemann P., Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994 Feb 25;269(8):6140–6148. [PubMed] [Google Scholar]
- Jordan N. J., Watson M. L., Westwick J. The protein phosphatase inhibitor calyculin A stimulates chemokine production by human synovial cells. Biochem J. 1995 Oct 1;311(Pt 1):89–95. doi: 10.1042/bj3110089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyoshi Y., Dörschner A., Mallet A. I., Christophers E., Schröder J. M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992 Aug 1;176(2):587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Harlow L. A., Johnson B., Evanoff H. L., Haines G. K., Burdick M. D., Pope R. M., Strieter R. M. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992 Sep;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardini J. B. Paradoxical stimulatory effect of the kinase inhibitor chelerythrine on the phosphorylation of a approximately 20 K M(r) protein present in the mitochondrial fraction of rat retina. Brain Res. 1995 Mar 6;673(2):194–198. doi: 10.1016/0006-8993(94)01369-s. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993 May 5;268(13):9194–9197. [PubMed] [Google Scholar]
- Mukaida N., Mahe Y., Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990 Dec 5;265(34):21128–21133. [PubMed] [Google Scholar]
- Palmer D. G., Hogg N., Revell P. A. Lymphocytes, polymorphonuclear leukocytes, macrophages and platelets in synovium involved by rheumatoid arthritis. A study with monoclonal antibodies. Pathology. 1986 Oct;18(4):431–437. doi: 10.3109/00313028609087564. [DOI] [PubMed] [Google Scholar]
- Rathanaswami P., Hachicha M., Sadick M., Schall T. J., McColl S. R. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993 Mar 15;268(8):5834–5839. [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Guesdon F. Interleukin 1 and tumor necrosis factor signal transduction mechanisms: potential targets for pharmacological control of inflammation. J Rheumatol Suppl. 1992 Jan;32:65–70. [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Toy K. J., Goeddel D. V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Taylor D. J., Evanson J. M., Woolley D. E. Contrasting effects of the protein kinase C inhibitor, staurosporine, on cytokine and phorbol ester stimulation of fructose 2,6-bisphosphate and prostaglandin E production by fibroblasts in vitro. Comparative studies using interleukin-1 alpha, tumour necrosis factor alpha, transforming growth factor beta, interferon-gamma and 12-O-tetradecanoylphorbol 13-acetate. Biochem J. 1990 Aug 1;269(3):573–577. doi: 10.1042/bj2690573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Ueda A., Okuda K., Ohno S., Shirai A., Igarashi T., Matsunaga K., Fukushima J., Kawamoto S., Ishigatsubo Y., Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994 Sep 1;153(5):2052–2063. [PubMed] [Google Scholar]
- Villiger P. M., Terkeltaub R., Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992 Jul 15;149(2):722–727. [PubMed] [Google Scholar]
- Watson M. L., Westwick J., Fincham N. J., Camp R. D. Elevation of PMN cytosolic free calcium and locomotion stimulated by novel peptides from IL-1-treated human synovial cell cultures. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1154–1160. doi: 10.1016/s0006-291x(88)81261-0. [DOI] [PubMed] [Google Scholar]
- Ways D. K., Cook P. P., Webster C., Parker P. J. Effect of phorbol esters on protein kinase C-zeta. J Biol Chem. 1992 Mar 5;267(7):4799–4805. [PubMed] [Google Scholar]
- Wilkinson S. E., Hallam T. J. Protein kinase C: is its pivotal role in cellular activation over-stated? Trends Pharmacol Sci. 1994 Feb;15(2):53–57. doi: 10.1016/0165-6147(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. E., Parker P. J., Nixon J. S. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993 Sep 1;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Robinson E. A., Tanaka S., Appella E., Kuratsu J., Leonard E. J. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989 Apr 1;169(4):1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Takeya M., Takahashi K., Kuratsu J., Leonard E. J. Production and characterization of mouse monoclonal antibodies against human monocyte chemoattractant protein-1. J Immunol. 1991 Oct 1;147(7):2229–2233. [PubMed] [Google Scholar]
- Zhang Q. Y., Hammerberg C., Baldassare J. J., Henderson P. A., Burns D., Ceska M., Voorhees J. J., Fisher G. J. Retinoic acid and phorbol ester synergistically up-regulate IL-8 expression and specifically modulate protein kinase C-epsilon in human skin fibroblasts. J Immunol. 1992 Aug 15;149(4):1402–1408. [PubMed] [Google Scholar]
- von Stebut E., Amon U., Herbert J. M., Wolff H. H. Investigations with the selective PKC inhibitor chelerythrine on human basophils. Agents Actions. 1994 Jun;41(Spec No):C56–C57. doi: 10.1007/BF02007765. [DOI] [PubMed] [Google Scholar]