Abstract
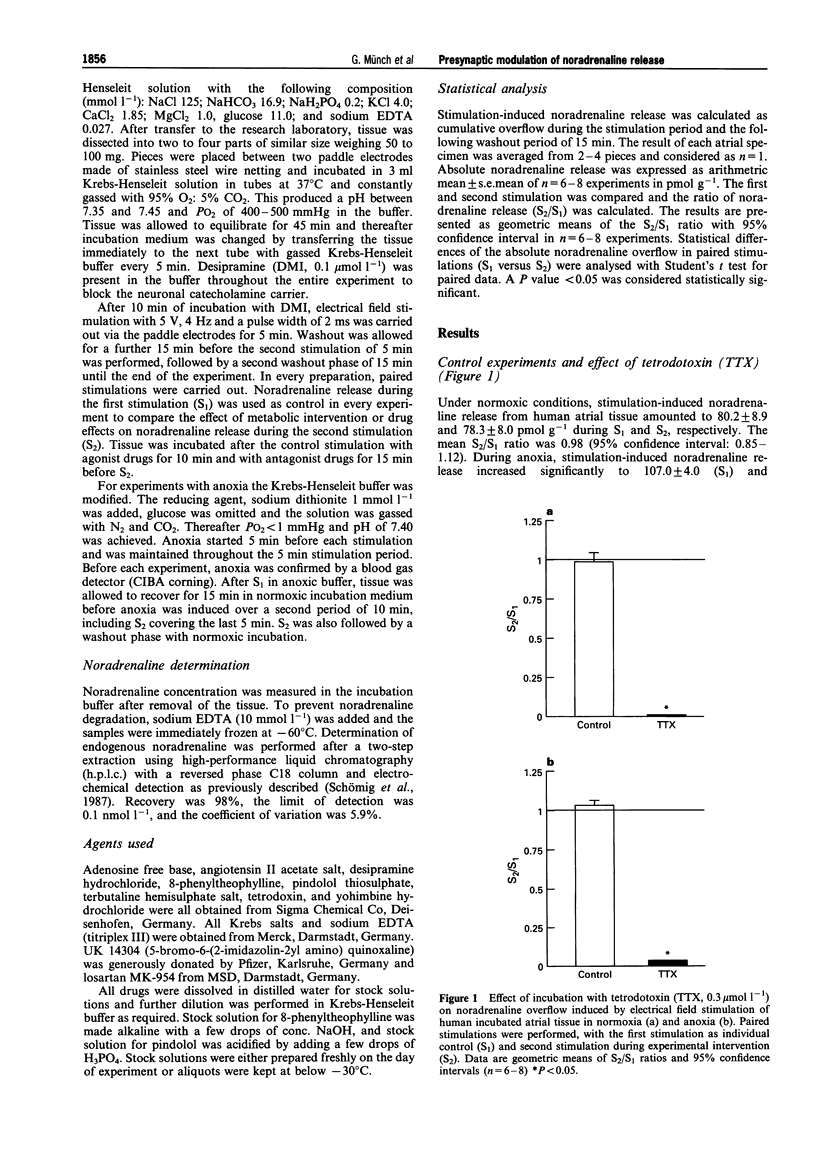
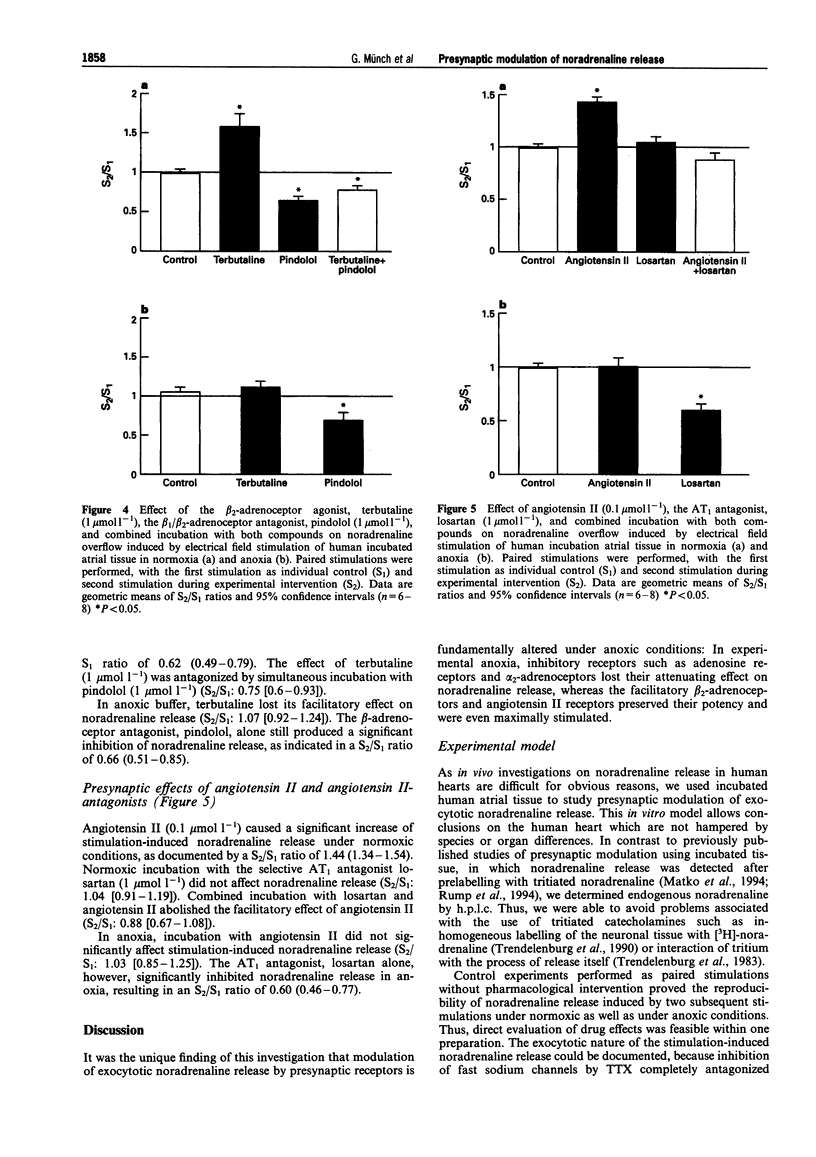
1. Presynaptic modulation of noradrenaline release in human atrial tissue specimens was investigated under normoxic and anoxic conditions. 2. Noradrenaline release was induced by electrical stimulation and release during experimental intervention (S2) was compared with release during a preceding control stimulation (S1). The results were expressed as the geometric means and 95% confidence intervals of the S2/S1 ratio. 3. The alpha 2-adrenoceptor agonist, UK 14304 (0.1 mumol-1) significantly inhibited noradrenaline release, resulting in a S2/S1 ratio of 0.49 (0.40-0.59), and the a 2-adrenoceptor antagonist, yohimbine (1 mumol l-1) increased noradrenaline release (S2/S1 1.83 [1.43-2.35]) during normoxia. Both compounds were ineffective during anoxia. 4. Adenosine (30 mumol-1) inhibited noradrenaline release with a S2/S1 ratio of 0.54 (0.42-0.66). The adenosine antagonist, 8-phenyltheophylline, alone had no effect during normoxia. During anoxia, neither adenosine nor 8-phenyltheophylline altered noradrenaline release. 5. The beta 2-adrenoceptor agonist, terbutaline (1 mumol l-1) increased (1.53 [1.14-2.01]) and the beta-adrenoceptor antagonist, pindolol (1 mumol l-1) suppressed noradrenaline release (0.62 [0.49-0.79]) under normoxic conditions. During anoxia, pindolol significantly inhibited noradrenaline release with a S2/S1 ratio of 0.66 (0.51-0.85), whereas terbutaline did not influence noradrenaline release. 6. Angiotensin II (0.1 mumol l-1 enhanced noradrenaline release resulting in a S2/S1 ratio of 1.44 (1.34-1.54), while the angiotensin II antagonist, losartan (1 mumol l-1) had no effect on noradrenaline release during normoxia. Conversely, angiotensin II did not increase noradrenaline release and losartan significantly inhibited noradrenaline release to a S2/S1 ratio of 0.60 (0.46-0.77) during anoxia. 7. In conclusion, human cardiac tissue possesses presynaptic inhibitory alpha 2-adrenoceptors and adenosine receptors, as well as facilitatory beta 2-adrenoceptors and angiotensin II receptors regulating noradrenaline release under normoxic conditions. During anoxia the responses to alpha 2-adrenoceptors and adenosine receptor stimulation are lost, whereas facilitatory responses to beta 2-adrenoceptors and adenosine II receptor stimulation are maintained and these receptors appear to be maximally stimulated. This differential presynaptic modulation in anoxia may contribute to enhanced sympathetic activity in ischaemia.
Full text
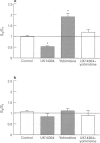
PDF
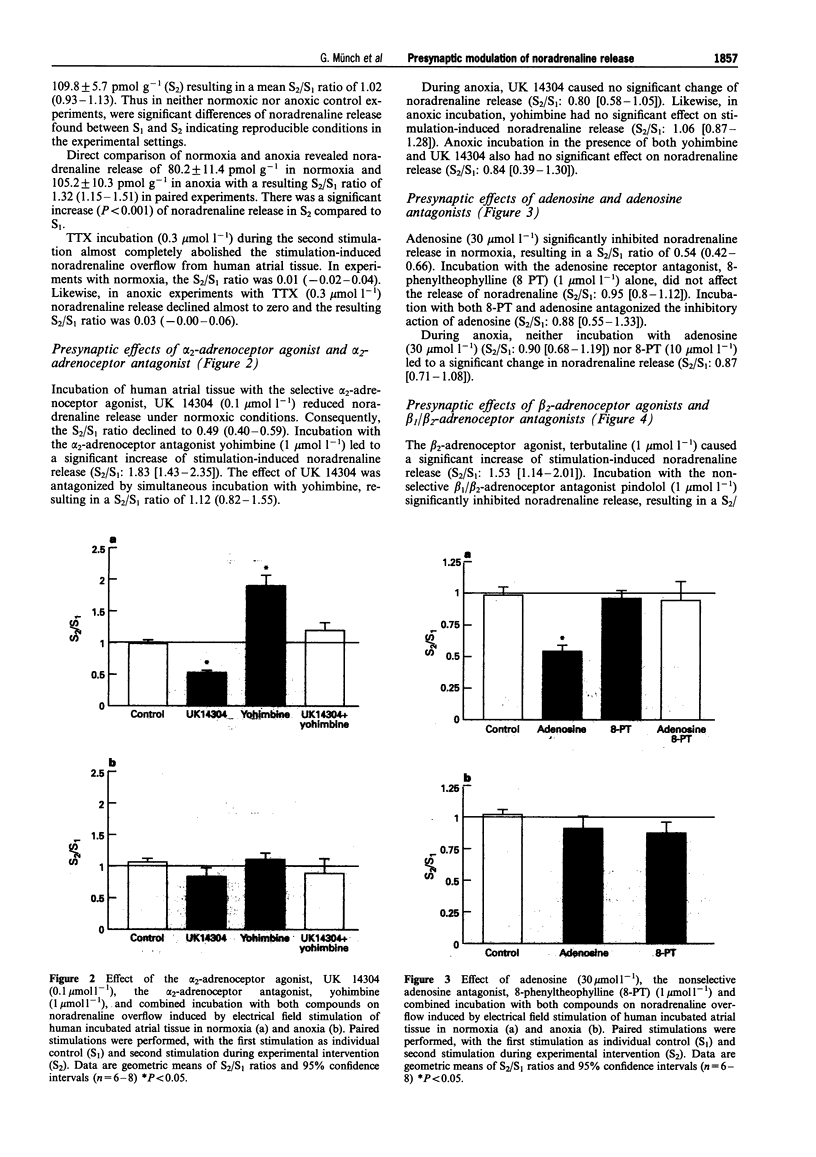



Images in this article
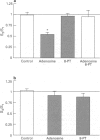
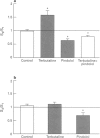
Selected References
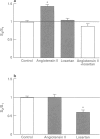
These references are in PubMed. This may not be the complete list of references from this article.
- Bohmann C., Schollmeyer P., Rump L. C. Methoxamine inhibits noradrenaline release through activation of alpha 1- and alpha 2-adrenoceptors in rat isolated kidney: involvement of purines and prostaglandins. Naunyn Schmiedebergs Arch Pharmacol. 1993 Mar;347(3):273–279. doi: 10.1007/BF00167445. [DOI] [PubMed] [Google Scholar]
- Brodde O. E. The functional importance of beta 1 and beta 2 adrenoceptors in the human heart. Am J Cardiol. 1988 Aug 11;62(5):24C–29C. doi: 10.1016/s0002-9149(88)80063-8. [DOI] [PubMed] [Google Scholar]
- Butterfield M. C., Chess-Williams R. Enhanced alpha-adrenoceptor responsiveness and receptor number during global ischaemia in the Langendorff perfused rat heart. Br J Pharmacol. 1990 Jul;100(3):641–645. doi: 10.1111/j.1476-5381.1990.tb15860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr E. A., Jr, Carroll M., Counsell R. E., Tyson J. W. Studies of uptake of the bretylium analogue, iodobenzyltrimethylammonium iodide, by non-primate, monkey and human hearts. Br J Clin Pharmacol. 1979 Nov;8(5):425–432. doi: 10.1111/j.1365-2125.1979.tb01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Nunoki K., Lai Y., De Jongh K., Thomsen W., Rossie S. Structure and modulation of voltage-sensitive sodium and calcium channels. Adv Second Messenger Phosphoprotein Res. 1990;24:30–35. [PubMed] [Google Scholar]
- Corr P. B., Gillis R. A. Autonomic neural influences on the dysrhythmias resulting from myocardial infarction. Circ Res. 1978 Jul;43(1):1–9. doi: 10.1161/01.res.43.1.1. [DOI] [PubMed] [Google Scholar]
- Dart A. M., Schömig A., Dietz R., Mayer E., Kübler W. Release of endogenous catecholamines in the ischemic myocardium of the rat. Part B: Effect of sympathetic nerve stimulation. Circ Res. 1984 Nov;55(5):702–706. doi: 10.1161/01.res.55.5.702. [DOI] [PubMed] [Google Scholar]
- Du X. J., Dart A. M., Riemersma R. A., Oliver M. F. Failure of the cholinergic modulation of norepinephrine release during acute myocardial ischemia in the rat. Circ Res. 1990 Apr;66(4):950–956. doi: 10.1161/01.res.66.4.950. [DOI] [PubMed] [Google Scholar]
- Esler M., Jackman G., Leonard P., Skews H., Bobik A., Korner P. Effect of norepinephrine uptake blockers on norepinephrine kinetics. Clin Pharmacol Ther. 1981 Jan;29(1):12–20. doi: 10.1038/clpt.1981.3. [DOI] [PubMed] [Google Scholar]
- Itoh A., Miwa S., Koshimura K., Akiyama Y., Takagi Y., Yamagata S., Kikuchi H., Masaki T. Ischemia-induced changes in catecholamine release and their mechanisms: a study using cultured bovine adrenal chromaffin cells. Brain Res. 1994 Apr 18;643(1-2):266–275. doi: 10.1016/0006-8993(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Matkó I., Fehér E., Vizi E. S. Receptor mediated presynaptic modulation of the release of noradrenaline in human papillary muscle. Cardiovasc Res. 1994 May;28(5):700–704. doi: 10.1093/cvr/28.5.700. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Wong T. M., Buja L. M., Lefkowitz R. J., Willerson J. T. Beta adrenergic and muscarinic cholinergic receptors in canine myocardium. Effects of ischemia. J Clin Invest. 1979 Nov;64(5):1423–1428. doi: 10.1172/JCI109600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niroomand F., Bangert M., Beyer T., Rauch B. Reduced adenylyl cyclase inhibition by carbachol and GTP during acute myocardial ischaemia. J Mol Cell Cardiol. 1992 May;24(5):471–475. doi: 10.1016/0022-2828(92)91836-t. [DOI] [PubMed] [Google Scholar]
- Niroomand F., Weinbrenner C., Weis A., Bangert M., Schwencke C., Marquetant R., Beyer T., Strasser R. H., Kübler W., Rauch B. Impaired function of inhibitory G proteins during acute myocardial ischemia of canine hearts and its reversal during reperfusion and a second period of ischemia. Possible implications for the protective mechanism of ischemic preconditioning. Circ Res. 1995 May;76(5):861–870. doi: 10.1161/01.res.76.5.861. [DOI] [PubMed] [Google Scholar]
- Noda K., Sasaguri M., Ideishi M., Ikeda M., Arakawa K. Role of locally formed angiotensin II and bradykinin in the reduction of myocardial infarct size in dogs. Cardiovasc Res. 1993 Feb;27(2):334–340. doi: 10.1093/cvr/27.2.334. [DOI] [PubMed] [Google Scholar]
- Ordway G. A., Jaconetta S. M., Halaris A. E. Characterization of subtypes of alpha-2 adrenoceptors in the human brain. J Pharmacol Exp Ther. 1993 Feb;264(2):967–976. [PubMed] [Google Scholar]
- Pfeffer M. A., Lamas G. A., Vaughan D. E., Parisi A. F., Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988 Jul 14;319(2):80–86. doi: 10.1056/NEJM198807143190204. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V., Friedel N., Heymann A., Bauer P., Neuss M., Rolfs A., Steffen C., Hildebrandt A., Hetzer R., Fleck E. Regulation, chamber localization, and subtype distribution of angiotensin II receptors in human hearts. Circulation. 1995 Mar 1;91(5):1461–1471. doi: 10.1161/01.cir.91.5.1461. [DOI] [PubMed] [Google Scholar]
- Richardt G., Waas W., Kranzhöfer R., Mayer E., Schömig A. Adenosine inhibits exocytotic release of endogenous noradrenaline in rat heart: a protective mechanism in early myocardial ischemia. Circ Res. 1987 Jul;61(1):117–123. doi: 10.1161/01.res.61.1.117. [DOI] [PubMed] [Google Scholar]
- Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985 Apr;17(4):291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- Rump L. C., Schwertfeger E., Schaible U., Fraedrich G., Schollmeyer P. Beta 2-adrenergic receptor and angiotensin II receptor modulation of sympathetic neurotransmission in human atria. Circ Res. 1994 Mar;74(3):434–440. doi: 10.1161/01.res.74.3.434. [DOI] [PubMed] [Google Scholar]
- Schrader J., Gerlach E. Compartmentation of cardiac adenine nucleotides and formation of adenosine. Pflugers Arch. 1976 Dec 28;367(2):129–135. doi: 10.1007/BF00585148. [DOI] [PubMed] [Google Scholar]
- Schömig A., Fischer S., Kurz T., Richardt G., Schömig E. Nonexocytotic release of endogenous noradrenaline in the ischemic and anoxic rat heart: mechanism and metabolic requirements. Circ Res. 1987 Feb;60(2):194–205. doi: 10.1161/01.res.60.2.194. [DOI] [PubMed] [Google Scholar]
- Schömig A., Richardt G., Kurz T. Sympatho-adrenergic activation of the ischemic myocardium and its arrhythmogenic impact. Herz. 1995 Jun;20(3):169–186. [PubMed] [Google Scholar]
- Seyfarth M., Feng Y., Hagl S., Sebening F., Richardt G., Schömig A. Effect of myocardial ischemia on stimulation-evoked noradrenaline release. Modulated neurotransmission in rat, guinea pig, and human cardiac tissue. Circ Res. 1993 Sep;73(3):496–502. doi: 10.1161/01.res.73.3.496. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Bao J. X., Gonon F., Msghina M., Stjärne E. On the geometry, kinetics and plasticity of sympathetic neuromuscular transmission. Jpn J Pharmacol. 1992;58 (Suppl 2):158P–165P. [PubMed] [Google Scholar]
- Strasser R. H., Braun-Dullaeus R., Walendzik H., Marquetant R. Alpha 1-receptor-independent activation of protein kinase C in acute myocardial ischemia. Mechanisms for sensitization of the adenylyl cyclase system. Circ Res. 1992 Jun;70(6):1304–1312. doi: 10.1161/01.res.70.6.1304. [DOI] [PubMed] [Google Scholar]
- Strasser R. H., Marquetant R. Supersensitivity of the adenylyl cyclase system in acute myocardial ischemia: evaluation of three independent mechanisms. Basic Res Cardiol. 1990;85 (Suppl 1):67–78. doi: 10.1007/978-3-662-11038-6_6. [DOI] [PubMed] [Google Scholar]
- Susanni E. E., Manders W. T., Knight D. R., Vatner D. E., Vatner S. F., Homcy C. J. One hour of myocardial ischemia decreases the activity of the stimulatory guanine-nucleotide regulatory protein Gs. Circ Res. 1989 Oct;65(4):1145–1150. doi: 10.1161/01.res.65.4.1145. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U., Stefano F. J., Grohmann M. The isotope effect of tritium in 3H-noradrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(2):128–140. doi: 10.1007/BF00634260. [DOI] [PubMed] [Google Scholar]