Abstract
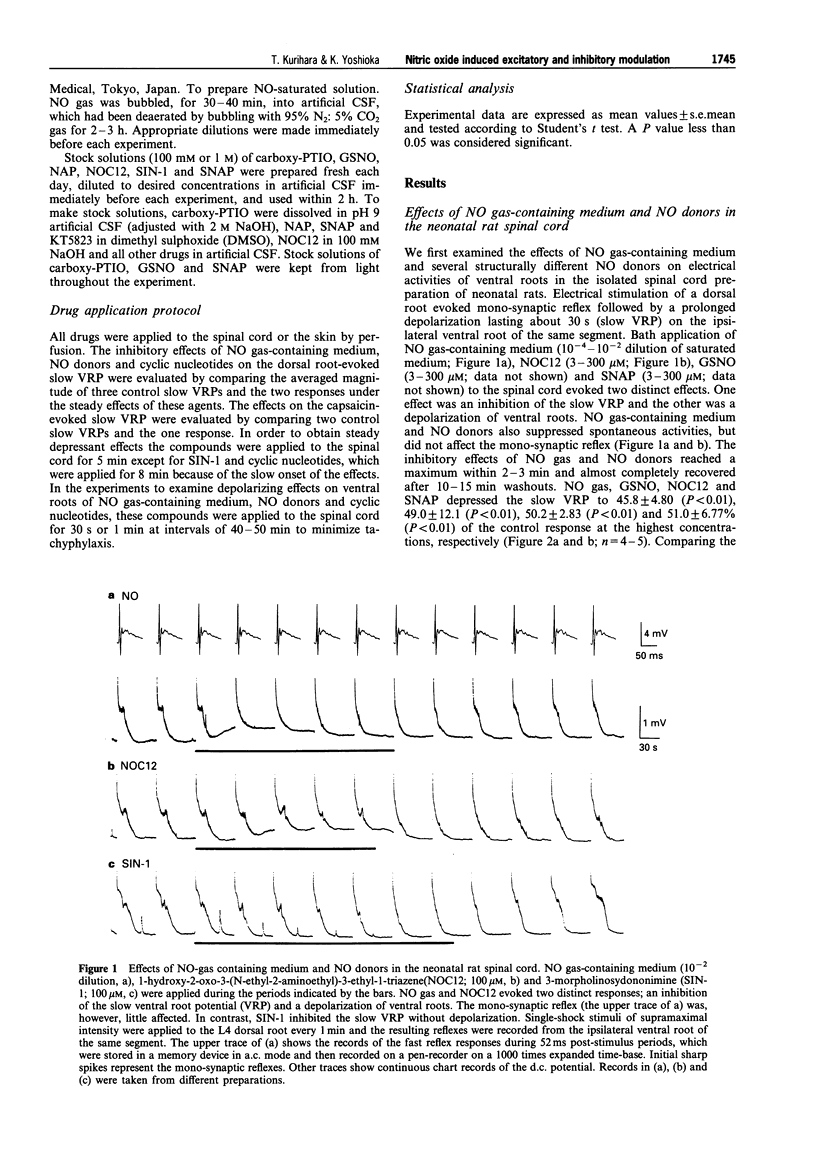
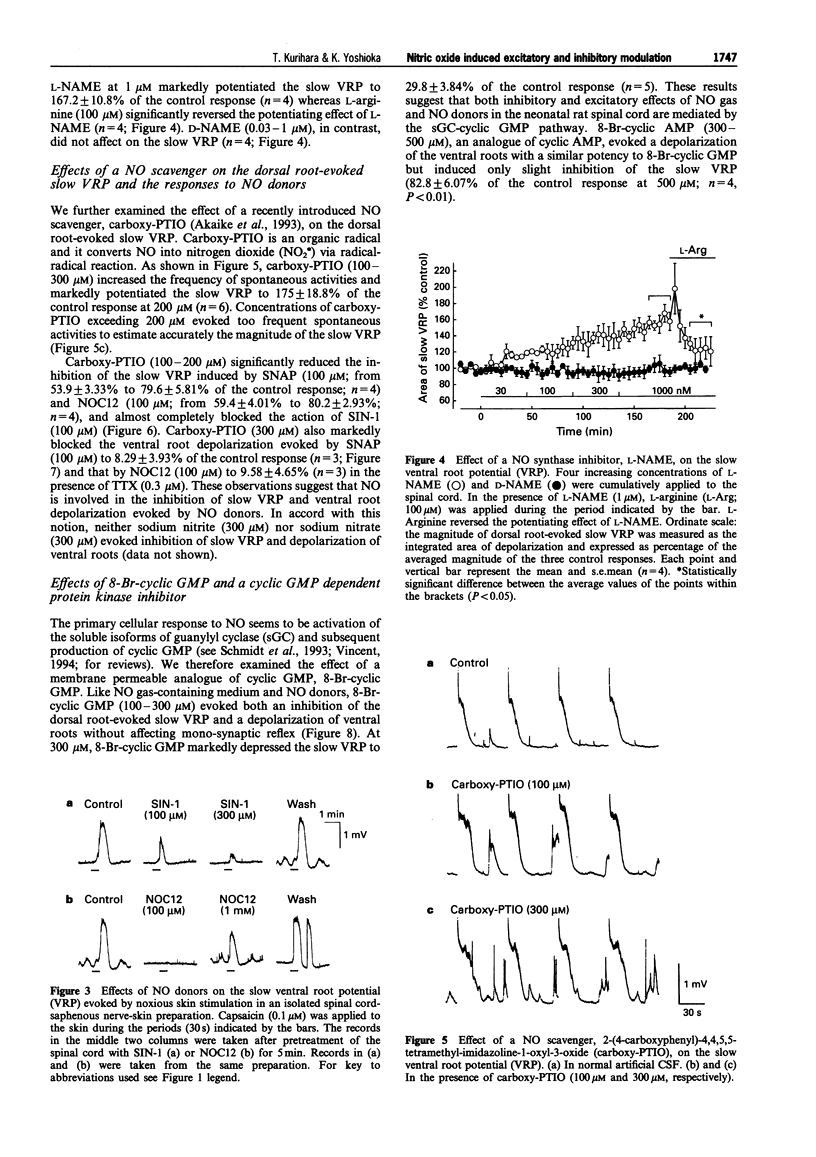
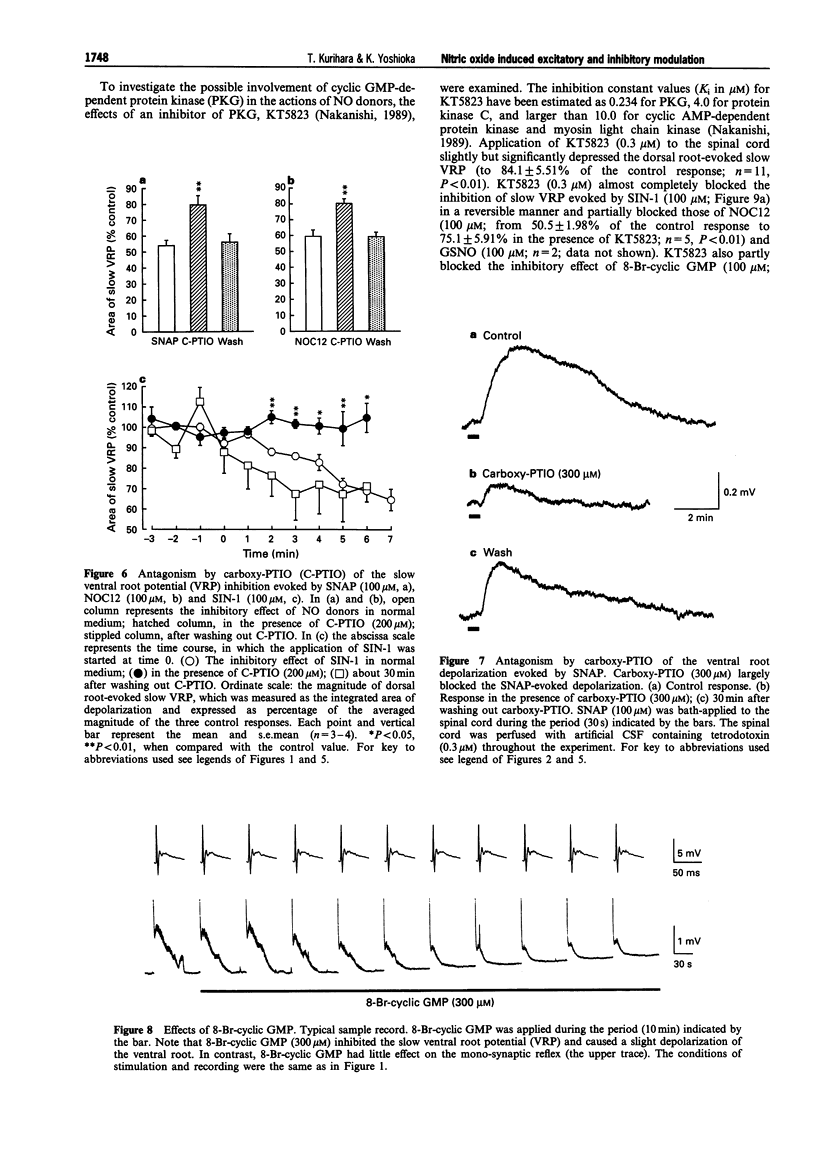
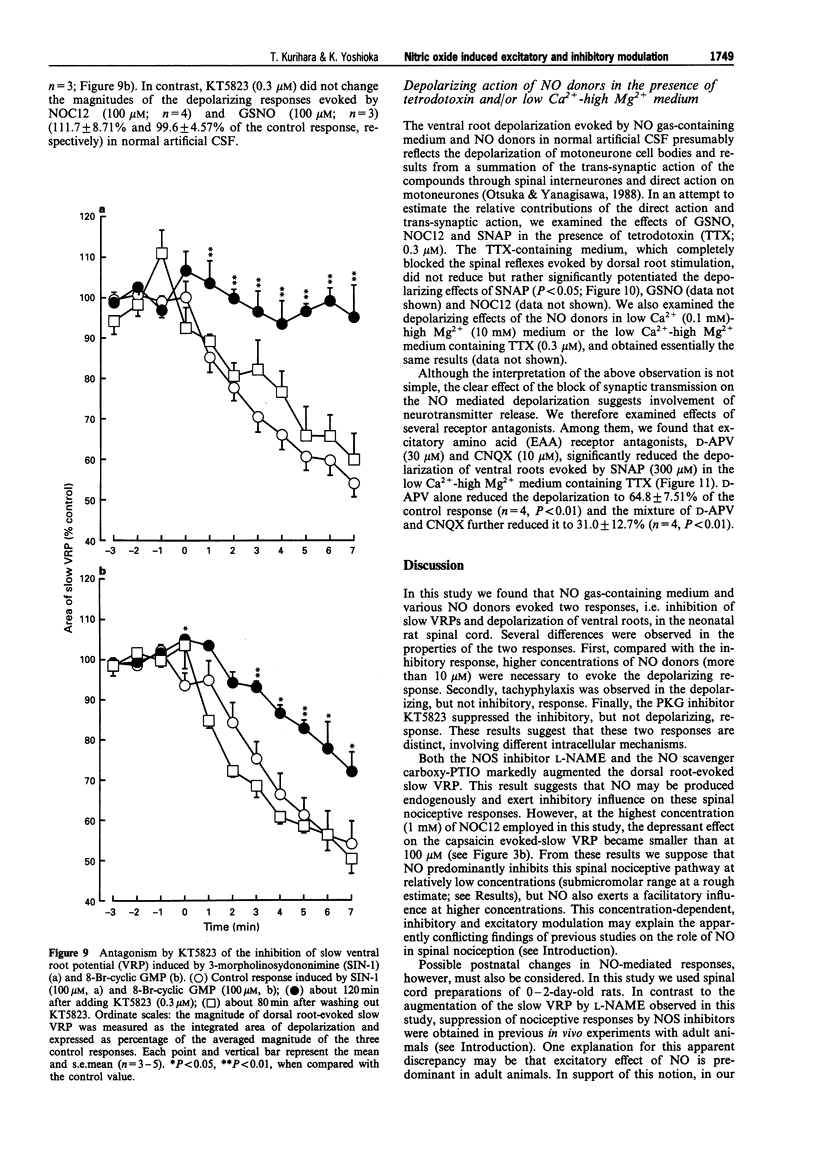
1. We investigated the role of nitric oxide (NO) in modulating spinal synaptic responses evoked by electrical and noxious sensory stimuli in the neonatal rat spinal cord in vitro. 2. Potentials were recorded extracellularly from a ventral root (L3-L5) of the isolated spinal cord preparation or spinal cord-saphenous nerve-skin preparation of 0- to 2-day-old rats. Spinal reflexes were elicited by electrical stimulation of the ipsilateral dorsal root or by noxious skin stimulation. 3. In the spinal cord preparation, single shock stimulation of a dorsal root at C-fibre strength induced mono-synaptic reflex followed by a slow depolarizing response lasting about 30 s (slow ventral root potential; slow VRP) in the ipsilateral ventral root of the same segment. Bath-application of NO gas-containing medium (10(-4)- 10(-2) dilution of saturated medium) and NO donors, 1-hydroxy-2-oxo-3-(N-ethyl-2-aminoethyl)-3-ethyl-1-triazene (NOC12, 3-300 microM), S-nitroso-N-acetyl-D,L-penicillamine (SNAP, 3-300 microM) and S-nitroso-L-glutathione (GSNO, 3-300 microM), produced an inhibition of the slow VRP and a depolarization of ventral roots. Another NO donor, 3-morpholinosydononimine (SIN-1, 30-300 microM), also depressed the slow VRP but did not depolarize ventral roots. These agents did not affect the mono-synaptic reflex. 4. In the spinal cord-saphenous nerve-skin preparation, application of capsaicin (0.1-0.2 microM) to skin evoked a slow depolarizing response of the L3 ventral root. This slow VRP was depressed by NOC12 (10-300 microM) and SIN-1 (100-300 microM). When the concentration of NOC12 was increased to 1 mM, spontaneous synaptic activities were augmented and the depressant effect of NOC12 on the slow VRP became less pronounced. 5. A NO-scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide( carboxy- PTIO, 100-300 microM) prevented the depressant effect on the dorsal root-evoked slow VRP and ventral root depolarizing effects of NO donors. Carboxy-PTIO increased spontaneous synaptic activities and markedly potentiated the slow VRP. A NO synthase (NOS) inhibitor, N omega-nitro-L-arginine methyl ester (L-NAME, 0.03-1 microM), but not D-NAME (0.03-1 microM), also markedly potentiated the slow VRP and this effect was reversed by L-arginine (300 microM). 6. 8-Bromo-cyclic guanosine 3': 5'-monophosphate (8-Br-cyclic GMP, 100-300 microM) produced both an inhibition of the slow VRP and a depolarization of ventral roots. A cyclic GMP-dependent protein kinase inhibitor, KT5823 (0.3 microM), partly inhibited the depressant effects of NO donors and 8-Br-cyclic GMP on the dorsal root-evoked slow VRP. In contrast, KT5823 did not inhibit the depolarizing effects of NO donors. 7. Perfusion of the spinal cord with medium containing tetrodotoxin (0.3 microM) and/or low Ca2+ (0.1 mM)-high Mg2+ (10 mM) markedly potentiated the depolarizing effect of NO donors. The SNAP-evoked depolarization in the tetrodotoxin-containing low Ca(2+)-high Mg2+ medium was significantly inhibited by excitatory amino acid receptor antagonists D-(-)-2-amino-5-phosphonovaleric acid (30 microM) and 6-cyano-7-nitroquinoxaline-2,3-dione (10 microM). 8. The present study suggests that inhibitory and excitatory mechanisms meditated by the NO-cyclic GMP cascade are involved in the primary afferent fibre-evoked nociceptive transmission in the neonatal rat spinal cord. The inhibitory mechanism, but not the excitatory mechanism, appears to be partly mediated by cyclic GMP-dependent protein kinase. It is also suggested that Ca(2+)-independent release of excitatory amino acid neurotransmitters contributes to the depolarizing response to NO of ventral roots.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi H., Konishi S., Otsuka M., Yanagisawa M. The role of substance P as a neurotransmitter in the reflexes of slow time courses in the neonatal rat spinal cord. Br J Pharmacol. 1985 Mar;84(3):663–673. doi: 10.1111/j.1476-5381.1985.tb16148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T., Yoshida M., Miyamoto Y., Sato K., Kohno M., Sasamoto K., Miyazaki K., Ueda S., Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993 Jan 26;32(3):827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- Bauer M. B., Murphy S., Gebhart G. F. Muscarinic cholinergic stimulation of the nitric oxide-cyclic GMP signaling system in cultured rat sensory neurons. Neuroscience. 1994 Sep;62(2):351–359. doi: 10.1016/0306-4522(94)90370-0. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994 Aug;13(2):301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- Böhme G. A., Bon C., Stutzmann J. M., Doble A., Blanchard J. C. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol. 1991 Jul 9;199(3):379–381. doi: 10.1016/0014-2999(91)90505-k. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Snyder S. H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994 Sep;14(9):5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Dun S. L., Wu S. Y., Förstermann U., Schmidt H. H., Tseng L. F. Nitric oxide synthase immunoreactivity in the rat, mouse, cat and squirrel monkey spinal cord. Neuroscience. 1993 Jun;54(4):845–857. doi: 10.1016/0306-4522(93)90579-5. [DOI] [PubMed] [Google Scholar]
- Dymshitz J., Vasko M. R. Nitric oxide and cyclic guanosine 3',5'-monophosphate do not alter neuropeptide release from rat sensory neurons grown in culture. Neuroscience. 1994 Oct;62(4):1279–1286. doi: 10.1016/0306-4522(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Erneux C., Couchie D., Dumont J. E., Baraniak J., Stec W. J., Abbad E. G., Petridis G., Jastorff B. Specificity of cyclic GMP activation of a multi-substrate cyclic nucleotide phosphodiesterase from rat liver. Eur J Biochem. 1981 Apr;115(3):503–510. doi: 10.1111/j.1432-1033.1981.tb06231.x. [DOI] [PubMed] [Google Scholar]
- Feelisch M., Ostrowski J., Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S13–S22. [PubMed] [Google Scholar]
- Garry M. G., Richardson J. D., Hargreaves K. M. Sodium nitroprusside evokes the release of immunoreactive calcitonin gene-related peptide and substance P from dorsal horn slices via nitric oxide-dependent and nitric oxide-independent mechanisms. J Neurosci. 1994 Jul;14(7):4329–4337. doi: 10.1523/JNEUROSCI.14-07-04329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Boulton C. L. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Guo J. Z., Yoshioka K., Yanagisawa M., Hosoki R., Hagan R. M., Otsuka M. Depression of primary afferent-evoked responses by GR71251 in the isolated spinal cord of the neonatal rat. Br J Pharmacol. 1993 Nov;110(3):1142–1148. doi: 10.1111/j.1476-5381.1993.tb13933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J. E., Dickenson A. H., Schachter M. Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in the rat. Neuropharmacology. 1992 Mar;31(3):251–258. doi: 10.1016/0028-3908(92)90175-o. [DOI] [PubMed] [Google Scholar]
- Hogg N., Darley-Usmar V. M., Wilson M. T., Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992 Jan 15;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki R., Yanagisawa M., Guo J. Z., Yoshioka K., Maehara T., Otsuka M. Effects of RP 67580, a tachykinin NK1 receptor antagonist, on a primary afferent-evoked response of ventral roots in the neonatal rat spinal cord. Br J Pharmacol. 1994 Dec;113(4):1141–1146. doi: 10.1111/j.1476-5381.1994.tb17116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto E. T., Marion L. Pharmacologic evidence that spinal muscarinic analgesia is mediated by an L-arginine/nitric oxide/cyclic GMP cascade in rats. J Pharmacol Exp Ther. 1994 Nov;271(2):601–608. [PubMed] [Google Scholar]
- Kalb R. G., Agostini J. Molecular evidence for nitric oxide-mediated motor neuron development. Neuroscience. 1993 Nov;57(1):1–8. doi: 10.1016/0306-4522(93)90107-q. [DOI] [PubMed] [Google Scholar]
- Kitto K. F., Haley J. E., Wilcox G. L. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett. 1992 Dec 14;148(1-2):1–5. doi: 10.1016/0304-3940(92)90790-e. [DOI] [PubMed] [Google Scholar]
- Kreeger J. S., Kitto K. F., Larson A. A. Substance P N-terminal metabolites and nitric oxide mediate capsaicin-induced antinociception in the adult mouse. J Pharmacol Exp Ther. 1994 Dec;271(3):1281–1285. [PubMed] [Google Scholar]
- Kurihara T., Suzuki H., Yanagisawa M., Yoshioka K. Muscarinic excitatory and inhibitory mechanisms involved in afferent fibre-evoked depolarization of motoneurones in the neonatal rat spinal cord. Br J Pharmacol. 1993 Sep;110(1):61–70. doi: 10.1111/j.1476-5381.1993.tb13772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Liuzzi F. J., Wu W., Scoville S. A., Schinco F. P. Development of nitric oxide synthase expression in the superficial dorsal horn of the rat spinal cord. Exp Neurol. 1993 Jun;121(2):275–278. doi: 10.1006/exnr.1993.1096. [DOI] [PubMed] [Google Scholar]
- Lothe A., Li P., Tong C., Yoon Y., Bouaziz H., Detweiler D. J., Eisenach J. C. Spinal cholinergic alpha-2 adrenergic interactions in analgesia and hemodynamic control: role of muscarinic receptor subtypes and nitric oxide. J Pharmacol Exp Ther. 1994 Sep;270(3):1301–1306. [PubMed] [Google Scholar]
- Malmberg A. B., Yaksh T. L. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain. 1993 Sep;54(3):291–300. doi: 10.1016/0304-3959(93)90028-N. [DOI] [PubMed] [Google Scholar]
- Mayer B., Schrammel A., Klatt P., Koesling D., Schmidt K. Peroxynitrite-induced accumulation of cyclic GMP in endothelial cells and stimulation of purified soluble guanylyl cyclase. Dependence on glutathione and possible role of S-nitrosation. J Biol Chem. 1995 Jul 21;270(29):17355–17360. doi: 10.1074/jbc.270.29.17355. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Lewin G. R., Wall P. D. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993 Aug;3(4):602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- Meller S. T., Dykstra C., Gebhart G. F. Production of endogenous nitric oxide and activation of soluble guanylate cyclase are required for N-methyl-D-aspartate-produced facilitation of the nociceptive tail-flick reflex. Eur J Pharmacol. 1992 Apr 7;214(1):93–96. doi: 10.1016/0014-2999(92)90102-a. [DOI] [PubMed] [Google Scholar]
- Meller S. T., Gebhart G. F. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993 Feb;52(2):127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- Meller S. T., Pechman P. S., Gebhart G. F., Maves T. J. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience. 1992 Sep;50(1):7–10. doi: 10.1016/0306-4522(92)90377-e. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., Oluyomi A. O., Babbedge R. C., Wallace P., Hart S. L. L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br J Pharmacol. 1991 Jan;102(1):198–202. doi: 10.1111/j.1476-5381.1991.tb12153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaumer J. C., Yanagisawa M., Otsuka M. Pharmacological properties of a C-fibre response evoked by saphenous nerve stimulation in an isolated spinal cord-nerve preparation of the newborn rat. Br J Pharmacol. 1989 Oct;98(2):373–382. doi: 10.1111/j.1476-5381.1989.tb12607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Yanagisawa M. Effect of a tachykinin antagonist on a nociceptive reflex in the isolated spinal cord-tail preparation of the newborn rat. J Physiol. 1988 Jan;395:255–270. doi: 10.1113/jphysiol.1988.sp016917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S., Kidd G. J., Trapp B. D., Dawson T. M., Bredt D. S., Wilson D. A., Traystman R. J., Snyder S. H., Hanley D. F. Rat spinal cord neurons contain nitric oxide synthase. Neuroscience. 1994 Mar;59(2):447–456. doi: 10.1016/0306-4522(94)90608-4. [DOI] [PubMed] [Google Scholar]
- Saran M., Michel C., Bors W. Reaction of NO with O2-. implications for the action of endothelium-derived relaxing factor (EDRF). Free Radic Res Commun. 1990;10(4-5):221–226. doi: 10.3109/10715769009149890. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Lohmann S. M., Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993 Aug 18;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H. NO., CO and .OH. Endogenous soluble guanylyl cyclase-activating factors. FEBS Lett. 1992 Jul 27;307(1):102–107. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- Shibuki K., Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991 Jan 24;349(6307):326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- Soyguder Z., Schmidt H. H., Morris R. Postnatal development of nitric oxide synthase type 1 expression in the lumbar spinal cord of the rat: a comparison with the induction of c-fos in response to peripheral application of mustard oil. Neurosci Lett. 1994 Oct 24;180(2):188–192. doi: 10.1016/0304-3940(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Spike R. C., Todd A. J., Johnston H. M. Coexistence of NADPH diaphorase with GABA, glycine, and acetylcholine in rat spinal cord. J Comp Neurol. 1993 Sep 15;335(3):320–333. doi: 10.1002/cne.903350303. [DOI] [PubMed] [Google Scholar]
- Thompson S. W., Babbedge R., Levers T., Dray A., Urban L. No evidence for contribution of nitric oxide to spinal reflex activity in the rat spinal cord in vitro. Neurosci Lett. 1995 Mar 24;188(2):121–124. doi: 10.1016/0304-3940(95)11412-p. [DOI] [PubMed] [Google Scholar]
- Valtschanoff J. G., Weinberg R. J., Rustioni A. NADPH diaphorase in the spinal cord of rats. J Comp Neurol. 1992 Jul 8;321(2):209–222. doi: 10.1002/cne.903210204. [DOI] [PubMed] [Google Scholar]
- Valtschanoff J. G., Weinberg R. J., Rustioni A., Schmidt H. H. Nitric oxide synthase and GABA colocalize in lamina II of rat spinal cord. Neurosci Lett. 1992 Dec 14;148(1-2):6–10. doi: 10.1016/0304-3940(92)90791-5. [DOI] [PubMed] [Google Scholar]
- Vincent S. R. Nitric oxide: a radical neurotransmitter in the central nervous system. Prog Neurobiol. 1994 Jan;42(1):129–160. doi: 10.1016/0301-0082(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Hosoki R., Otsuka M. The isolated spinal cord-skin preparation of the newborn rat and effects of some algogenic and analgesic substances. Eur J Pharmacol. 1992 Sep 22;220(2-3):111–117. doi: 10.1016/0014-2999(92)90737-o. [DOI] [PubMed] [Google Scholar]
- Zhang X., Verge V., Wiesenfeld-Hallin Z., Ju G., Bredt D., Synder S. H., Hökfelt T. Nitric oxide synthase-like immunoreactivity in lumbar dorsal root ganglia and spinal cord of rat and monkey and effect of peripheral axotomy. J Comp Neurol. 1993 Sep 22;335(4):563–575. doi: 10.1002/cne.903350408. [DOI] [PubMed] [Google Scholar]
- Zhuo M., Meller S. T., Gebhart G. F. Endogenous nitric oxide is required for tonic cholinergic inhibition of spinal mechanical transmission. Pain. 1993 Jul;54(1):71–78. doi: 10.1016/0304-3959(93)90101-T. [DOI] [PubMed] [Google Scholar]


