Abstract
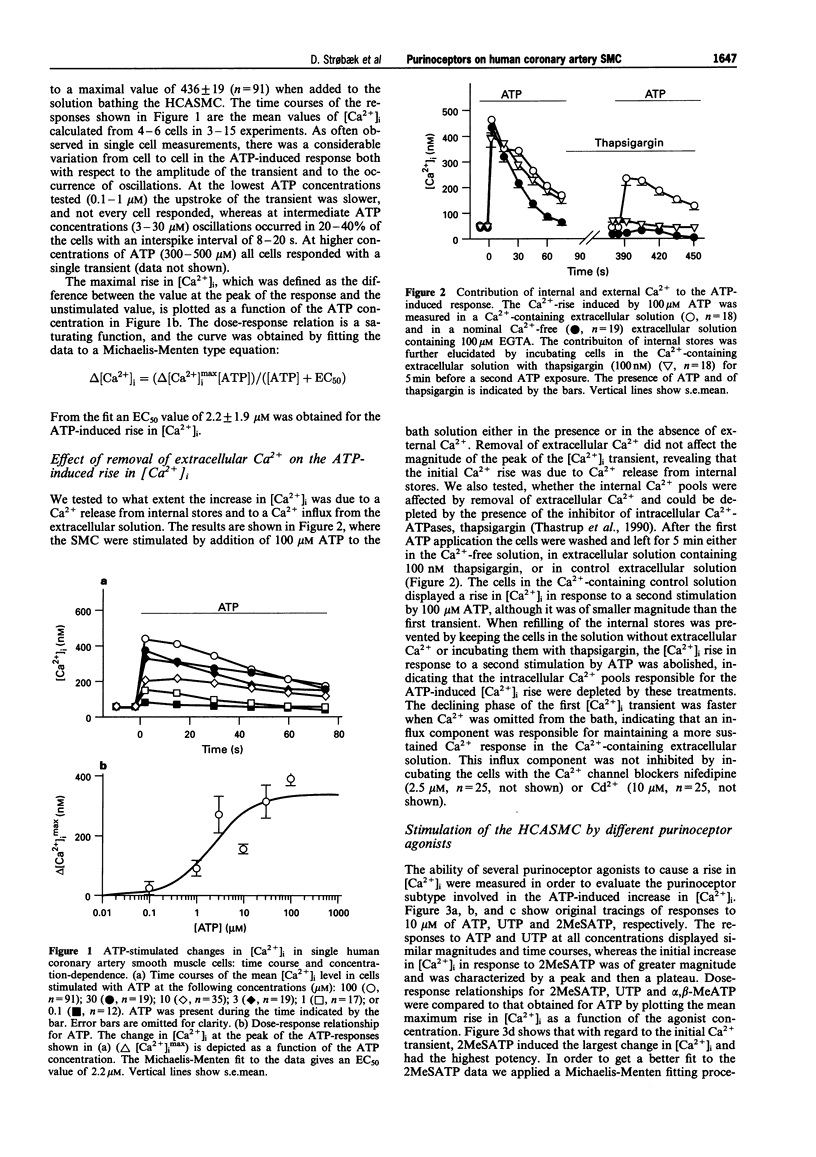
1. The effects of extracellular adenosine 5'-triphosphate (ATP) on smooth muscles are mediated by a variety of purinoceptors. In this study we addressed the identity of the purinoceptors on smooth muscle cells (SMC) cultured from human large coronary arteries. Purinoceptor-mediated increases in [Ca2+]i were measured in single fura-2 loaded cells by applying a digital imaging technique, and the formation of inositol phosphate compounds was quantified after separation on an anion exchange column. 2. Stimulation of the human coronary artery SMC (HCASMC) with extracellular ATP at concentrations of 0.1-100 microM induced a transient increase in [Ca2+]i from a resting level of 49 +/- 21 nM to a maximum of 436 +/- 19 nM. The effect was dose-dependent with an EC50 value for ATP of 2.2 microM. 3. The rise in [Ca2+]i was independent of the presence of external Ca2+, but was abolished after depletion of intracellular stores by incubation with 100 nM thapsigargin. 4. [Ca2+]i was measured upon stimulation of the cells with 0.1-100 microM of the more specific P2-purinoceptor agonists alpha, beta-methyleneadenosine 5'-triphosphate (alpha,beta-MeATP), 2-methylthioadenosine 5'-triphosphate (2MeSATP) and uridine 5'-triphosphate (UTP). alpha, beta-MeATP was without effect, whereas 2MeSATP and UTP induced release of Ca2+ from internal stores with 2MeSATP being the most potent agonist (EC50 = 0.17 microM), and UTP having a potency similar to ATP. The P1 purinoceptor agonist adenosine (100 microM) did not induce any changes in [Ca2+]i. 5. Stimulation with a submaximal concentration of UTP (10 microM) abolished a subsequent ATP-induced increase in [Ca2+]i, whereas an increase was induced by ATP after stimulation with 10 microM 2MeSATP. 6. The phospholipase C (PLC) inhibitor U73122 (5 microM) abolished the purinoceptor-activated rise in [Ca2+]i, whereas pretreatment with the Gi protein inhibitor pertussis toxin (PTX, 500 ng ml-1) was without effect on ATP-evoked [Ca2+]i increases. 7. Receptor activation with UTP and ATP resulted in formation of inositol phosphates with peak levels of inositol 1, 4, 5-trisphosphate (Ins(1, 4, 5)P3) observed 5-20 s after stimulation. 8. These findings show, that cultured HCASMC express G protein-coupled purinoceptors, which upon stimulation activate PLC to induce enhanced Ins(1, 4, 5)P3 production causing release of Ca2+ from internal stores. Since a release of Ca2+ was induced by 2MeSATP as well as by UTP, the data indicate that P2y- as well as P2U-purinoceptors are expressed by the HCASMC.
Full text
PDF

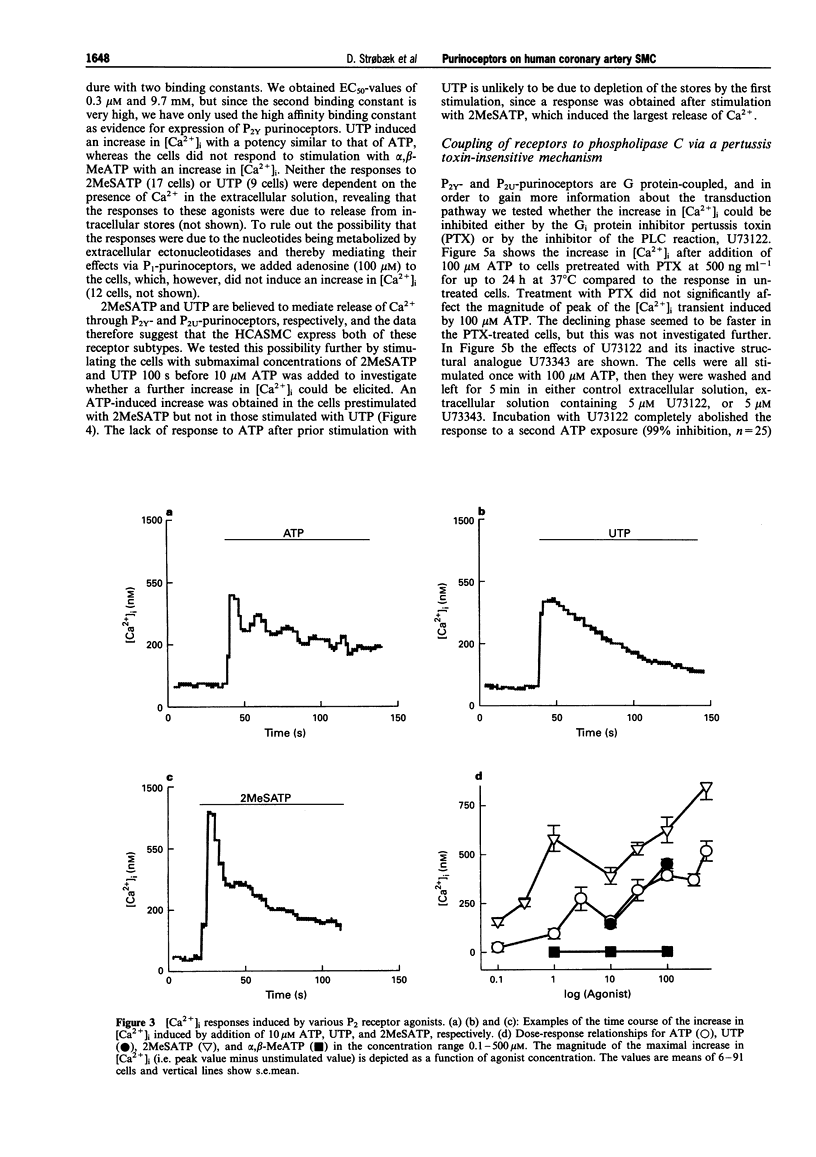


Selected References
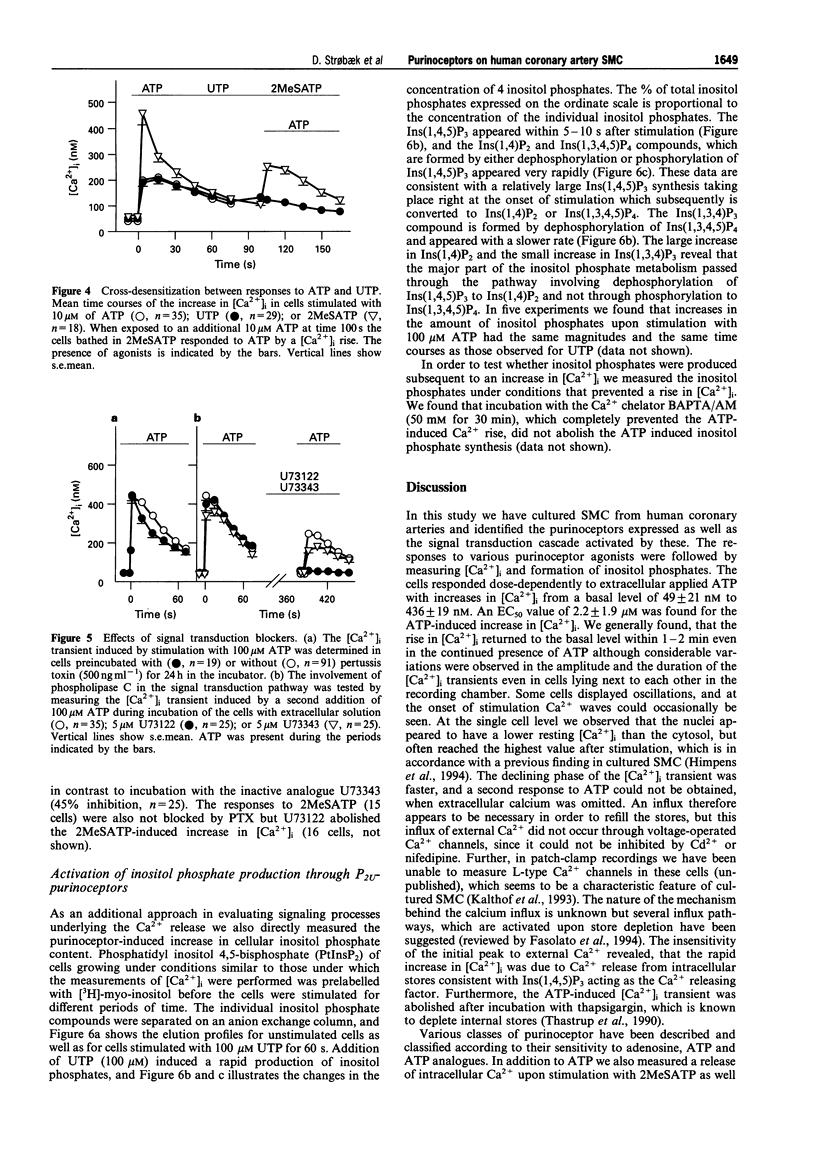
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard E. A., Burnstock G., Webb T. E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994 Mar;15(3):67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
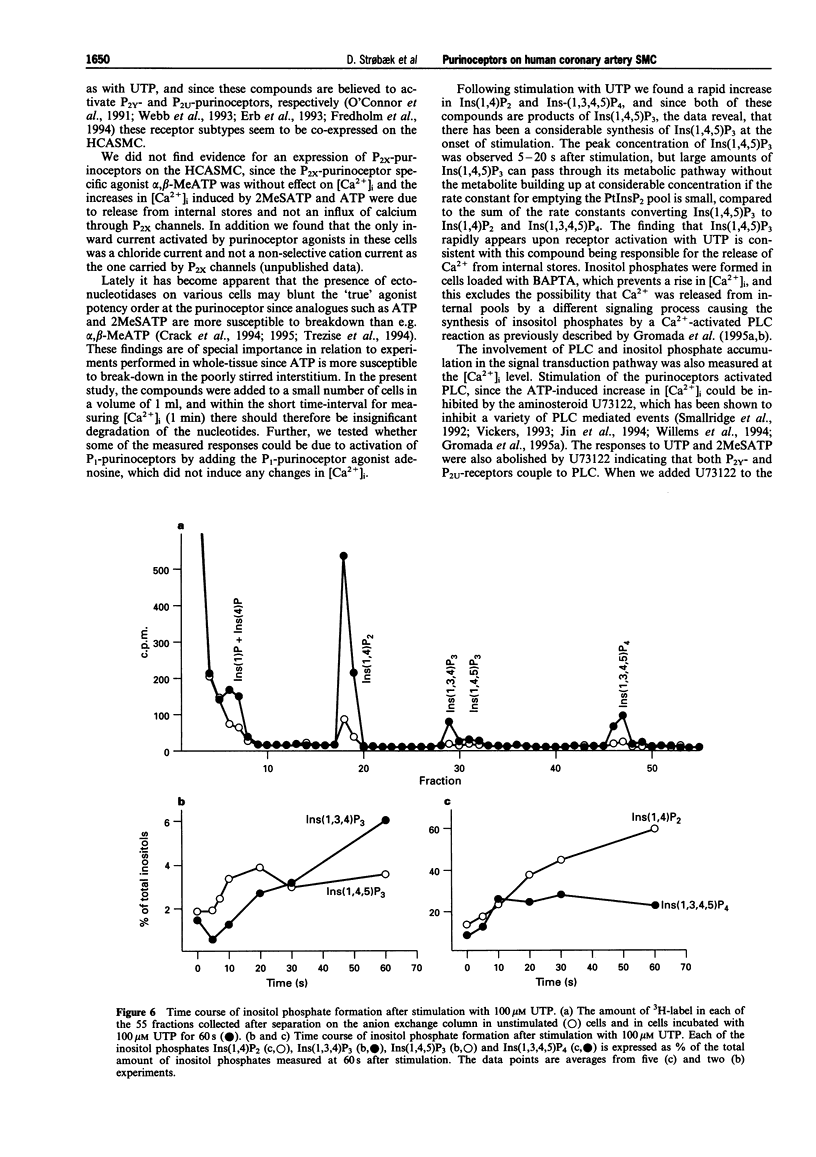
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Corr L., Burnstock G. Vasodilator response of coronary smooth muscle to the sympathetic co-transmitters noradrenaline and adenosine 5'-triphosphate. Br J Pharmacol. 1991 Oct;104(2):337–342. doi: 10.1111/j.1476-5381.1991.tb12432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack B. E., Beukers M. W., McKechnie K. C., Ijzerman A. P., Leff P. Pharmacological analysis of ecto-ATPase inhibition: evidence for combined enzyme inhibition and receptor antagonism in P2X-purinoceptor ligands. Br J Pharmacol. 1994 Dec;113(4):1432–1438. doi: 10.1111/j.1476-5381.1994.tb17157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack B. E., Pollard C. E., Beukers M. W., Roberts S. M., Hunt S. F., Ingall A. H., McKechnie K. C., IJzerman A. P., Leff P. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br J Pharmacol. 1995 Jan;114(2):475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Callewaert G., Declerck I., Casteels R. ATP-induced Ca2+ release and Cl- current in cultured smooth muscle cells from pig aorta. J Physiol. 1991;440:623–634. doi: 10.1113/jphysiol.1991.sp018728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Erb L., Lustig K. D., Sullivan D. M., Turner J. T., Weisman G. A. Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10449–10453. doi: 10.1073/pnas.90.22.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994 Mar;15(3):77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J., Jørgensen T. D., Dissing S. Role of protein kinase C in the regulation of inositol phosphate production and Ca2+ mobilization evoked by ATP and acetylcholine in rat lacrimal acini. Pflugers Arch. 1995 Feb;429(4):578–586. doi: 10.1007/BF00704164. [DOI] [PubMed] [Google Scholar]
- Gromada J., Jørgensen T. D., Dissing S. The release of intracellular Ca2+ in lacrimal acinar cells by alpha-, beta-adrenergic and muscarinic cholinergic stimulation: the roles of inositol triphosphate and cyclic ADP-ribose. Pflugers Arch. 1995 Apr;429(6):751–761. doi: 10.1007/BF00374798. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Himpens B., De Smedt H., Casteels R. Relationship between [Ca2+] changes in nucleus and cytosol. Cell Calcium. 1994 Oct;16(4):239–246. doi: 10.1016/0143-4160(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Jin W., Lo T. M., Loh H. H., Thayer S. A. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994 Apr 11;642(1-2):237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- Kalthof B., Bechem M., Flocke K., Pott L., Schramm M. Kinetics of ATP-induced Ca2+ transients in cultured pig aortic smooth muscle cells depend on ATP concentration and stored Ca2+. J Physiol. 1993 Jul;466:245–262. [PMC free article] [PubMed] [Google Scholar]
- O'Connor S. E., Dainty I. A., Leff P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci. 1991 Apr;12(4):137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fernandez M., Katz G. M., Suarez-Kurtz G., Kaczorowski G. J., Reuben J. P. Mobilization of intracellular calcium in cultured vascular smooth muscle cells by uridine triphosphate and the calcium ionophore A23187. J Membr Biol. 1993 Sep;135(3):273–287. doi: 10.1007/BF00211099. [DOI] [PubMed] [Google Scholar]
- Smallridge R. C., Kiang J. G., Gist I. D., Fein H. G., Galloway R. J. U-73122, an aminosteroid phospholipase C antagonist, noncompetitively inhibits thyrotropin-releasing hormone effects in GH3 rat pituitary cells. Endocrinology. 1992 Oct;131(4):1883–1888. doi: 10.1210/endo.131.4.1396332. [DOI] [PubMed] [Google Scholar]
- Tawada Y., Furukawa K., Shigekawa M. ATP-induced calcium transient in cultured rat aortic smooth muscle cells. J Biochem. 1987 Dec;102(6):1499–1509. doi: 10.1093/oxfordjournals.jbchem.a122197. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezise D. J., Bell N. J., Kennedy I., Humphrey P. P. Effects of divalent cations on the potency of ATP and related agonists in the rat isolated vagus nerve: implications for P2 purinoceptor classification. Br J Pharmacol. 1994 Oct;113(2):463–470. doi: 10.1111/j.1476-5381.1994.tb17012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994 Oct 6;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vickers J. D. U73122 affects the equilibria between the phosphoinositides as well as phospholipase C activity in unstimulated and thrombin-stimulated human and rabbit platelets. J Pharmacol Exp Ther. 1993 Sep;266(3):1156–1163. [PubMed] [Google Scholar]
- Webb T. E., Simon J., Krishek B. J., Bateson A. N., Smart T. G., King B. F., Burnstock G., Barnard E. A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993 Jun 14;324(2):219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Willems P. H., Van de Put F. H., Engbersen R., Bosch R. R., Van Hoof H. J., de Pont J. J. Induction of Ca2+ oscillations by selective, U73122-mediated, depletion of inositol-trisphosphate-sensitive Ca2+ stores in rabbit pancreatic acinar cells. Pflugers Arch. 1994 Jun;427(3-4):233–243. doi: 10.1007/BF00374529. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol Sci. 1991 Sep;12(9):319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]
- von der Weid P. Y., Serebryakov V. N., Orallo F., Bergmann C., Snetkov V. A., Takeda K. Effects of ATP on cultured smooth muscle cells from rat aorta. Br J Pharmacol. 1993 Mar;108(3):638–645. doi: 10.1111/j.1476-5381.1993.tb12854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


