Abstract
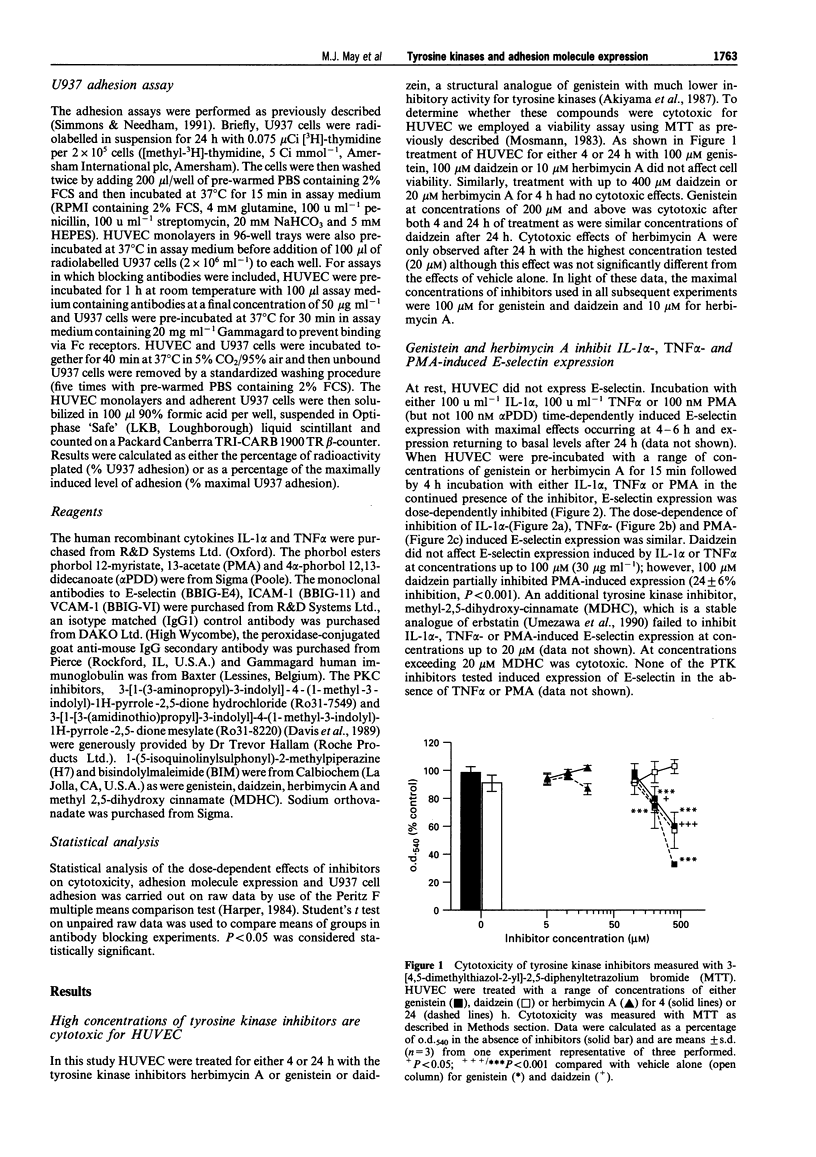
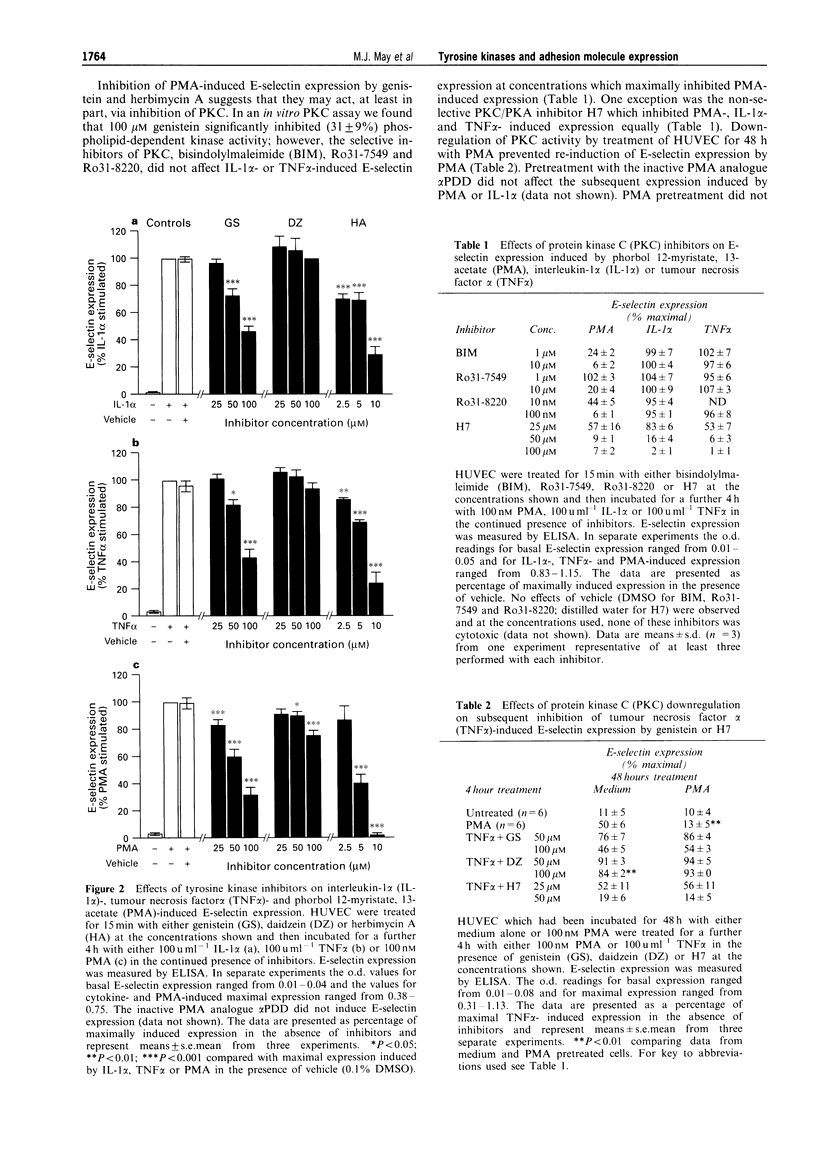
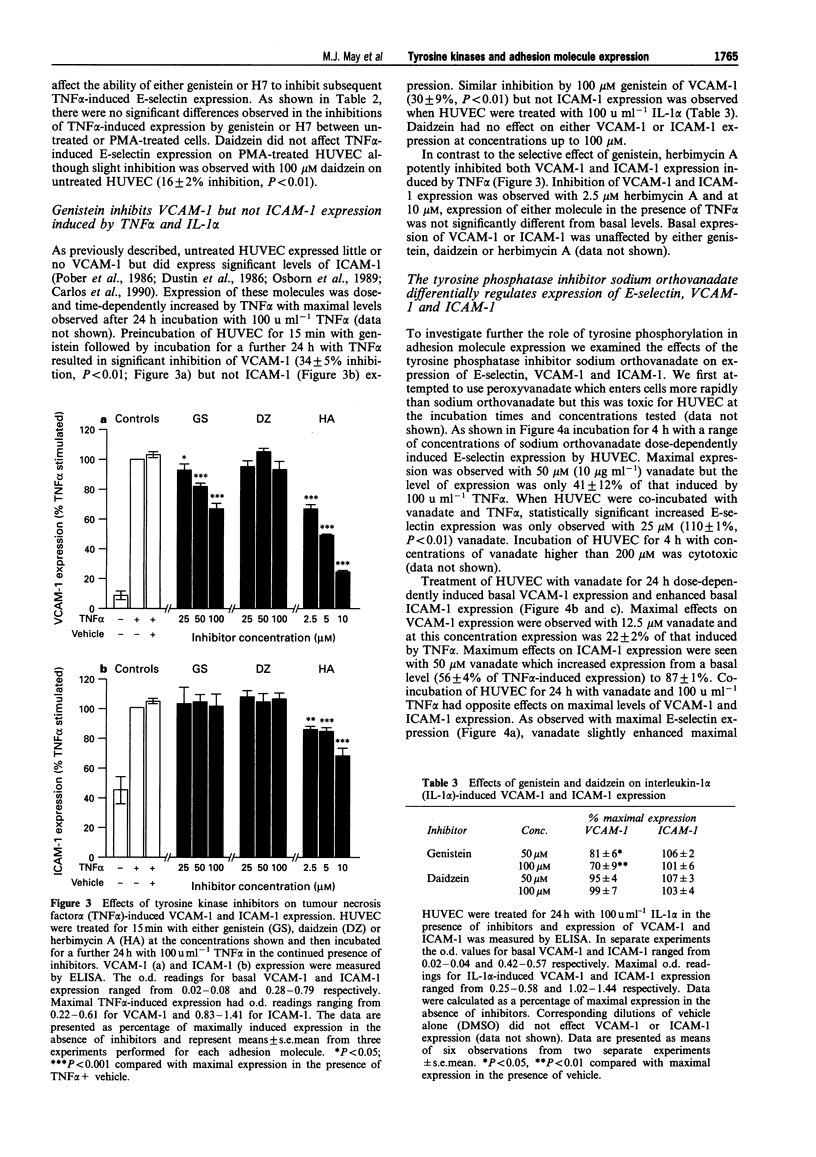
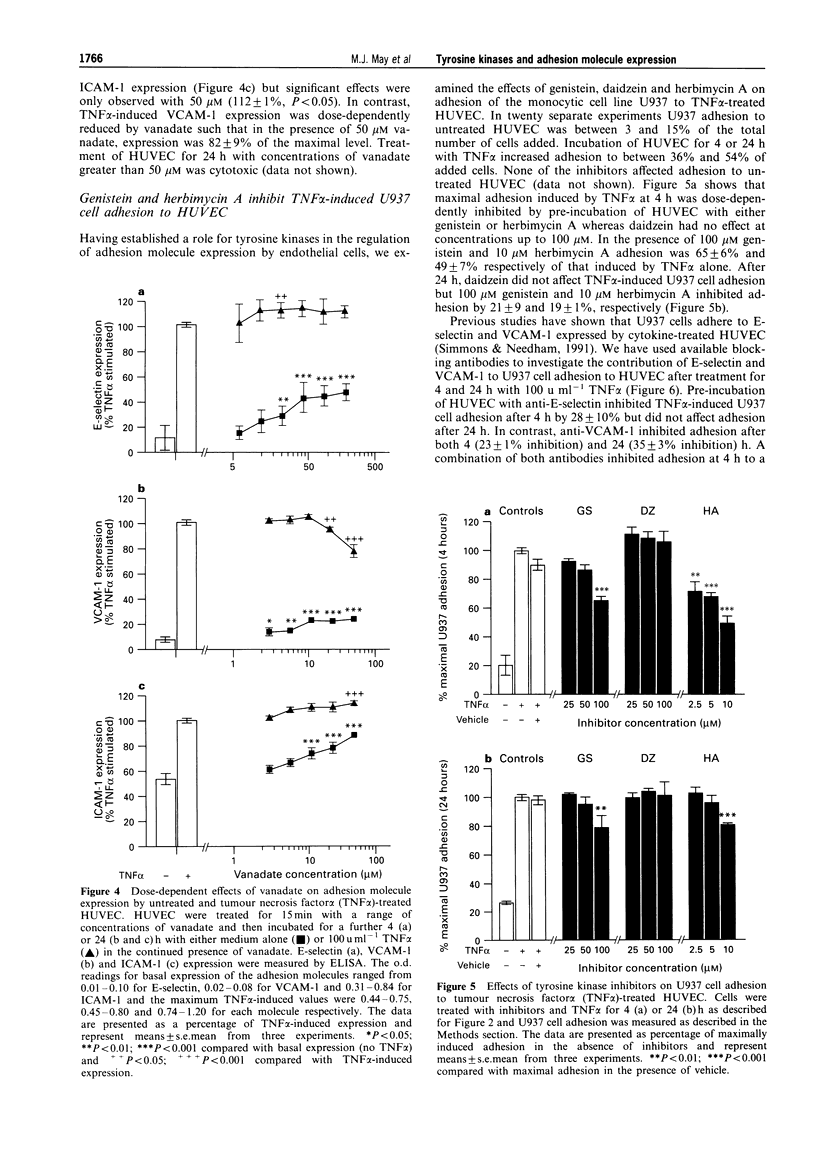
1. Endothelial cells can be stimulated by the pro-inflammatory cytokines interleukin (IL)-1 alpha and tumour necrosis factor (TNF) alpha to express the leukocyte adhesion molecules E-selectin, vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 but the intracellular signalling mechanisms leading to this expression are incompletely understood. We have investigated the role of protein tyrosine kinases (PTK) in adhesion molecule expression by cytokine-activated human umbilical vein endothelial cells (HUVEC) using the PTK inhibitors genistein and herbimycin A, and the protein tyrosine phosphatase (PTP) inhibitor sodium orthovanadate. 2. Maximal E-selectin expression induced by incubation of HUVEC for 4 h with IL-1 alpha (100 u ml-1) and TNF alpha (100 u ml-1) was dose-dependently inhibited by genistein and herbimycin A. Although similar effects were seen on phorbol 12-myristate, 13-acetate (PMA)-induced expression, this was not due to inhibition of protein kinase C (PKC) activity as the selective inhibitors of PKC, bisindolylmaleimide (BIM), Ro31-7549 or Ro31-8220 did not affect IL-1 alpha- or TNF alpha-induced E-selectin expression at concentrations which maximally inhibited PMA-induced expression. 3. Genistein inhibited VCAM-1 expression induced by incubation of HUVEC for 24 h with TNF alpha or IL-1 alpha whereas it did not affect ICAM-1 expression induced by 24 h incubation with either of these cytokines. Herbimycin A inhibited both VCAM-1 and ICAM-1 expression induced by TNF alpha. 4. Basal expression of E-selectin, VCAM-1 and ICAM-1 was dose-dependently enhanced by sodium orthovanadate. In contrast, vanadate differentially affected TNF alpha-induced expression of these molecules with maximal E-selectin and ICAM-1 expression being slightly enhanced and VCAM-1 expression dose-dependently reduced. 5. We also studied the effects of PTK and PTP inhibitors on adhesion of the human pre-myeloid cell line U937 to TNF alpha-stimulated HUVEC. Adhesion of U937 cells to HUVEC pretreated for 4 or 24 h with TNF alpha was dose-dependently inhibited by genistein and herbimycin A but unaffected by daidzein. Adhesion of U937 cells after 4 h was partially inhibited by blocking antibodies against both E-selectin and VCAM-1 but after 24 h was only inhibited by anti-VCAM-1. 6. Sodium orthovanadate had no effect on TNF alpha-induced U937 adhesion but dose-dependently enhanced adhesion to unstimulated HUVEC. Vanadate-induced adhesion was inhibited by an antibody against VCAM-1. 7. These results demonstrate that PTK-mediated phosphorylation events are important for the regulation of adhesion molecule expression by human endothelial cells, and additionally show that PTK inhibitors differentially affect upregulation of different adhesion molecules, implicating divergent regulatory pathways for cytokine-induced adhesion molecule expression.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Alon R., Kassner P. D., Carr M. W., Finger E. B., Hemler M. E., Springer T. A. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995 Mar;128(6):1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. A., Sleath P. R., deRoos P. C., Dower S. K., Virca G. D. Interleukin-1 represents a new modality for the activation of extracellular signal-regulated kinases/microtubule-associated protein-2 kinases. J Biol Chem. 1991 Nov 25;266(33):22661–22670. [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Chan B. M., Elices M. J., Murphy E., Hemler M. E. Adhesion to vascular cell adhesion molecule 1 and fibronectin. Comparison of alpha 4 beta 1 (VLA-4) and alpha 4 beta 7 on the human B cell line JY. J Biol Chem. 1992 Apr 25;267(12):8366–8370. [PubMed] [Google Scholar]
- Collins T. Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest. 1993 May;68(5):499–508. [PubMed] [Google Scholar]
- Corbett J. A., Kwon G., Misko T. P., Rodi C. P., McDaniel M. L. Tyrosine kinase involvement in IL-1 beta-induced expression of iNOS by beta-cells purified from islets of Langerhans. Am J Physiol. 1994 Jul;267(1 Pt 1):C48–C54. doi: 10.1152/ajpcell.1994.267.1.C48. [DOI] [PubMed] [Google Scholar]
- David M., Grimley P. M., Finbloom D. S., Larner A. C. A nuclear tyrosine phosphatase downregulates interferon-induced gene expression. Mol Cell Biol. 1993 Dec;13(12):7515–7521. doi: 10.1128/mcb.13.12.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- De Luca L. G., Johnson D. R., Whitley M. Z., Collins T., Pober J. S. cAMP and tumor necrosis factor competitively regulate transcriptional activation through and nuclear factor binding to the cAMP-responsive element/activating transcription factor element of the endothelial leukocyte adhesion molecule-1 (E-selectin) promoter. J Biol Chem. 1994 Jul 29;269(30):19193–19196. [PubMed] [Google Scholar]
- Deisher T. A., Haddix T. L., Montgomery K. F., Pohlman T. H., Kaushansky K., Harlan J. M. The role of protein kinase C in the induction of VCAM-1 expression on human umbilical vein endothelial cells. FEBS Lett. 1993 Oct 4;331(3):285–290. doi: 10.1016/0014-5793(93)80354-w. [DOI] [PubMed] [Google Scholar]
- Deisher T. A., Sato T. T., Pohlman T. H., Harlan J. M. A protein kinase C agonist, selective for the beta I isozyme, induces E-selectin and VCAM-1 expression on HUVEC but does not translocate PKC. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1283–1290. doi: 10.1006/bbrc.1993.1764. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Fuortes M., Jin W. W., Nathan C. Adhesion-dependent protein tyrosine phosphorylation in neutrophils treated with tumor necrosis factor. J Cell Biol. 1993 Feb;120(3):777–784. doi: 10.1083/jcb.120.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galéa P., Thibault G., Lacord M., Bardos P., Lebranchu Y. IL-4, but not tumor necrosis factor-alpha, increases endothelial cell adhesiveness for lymphocytes by activating a cAMP-dependent pathway. J Immunol. 1993 Jul 15;151(2):588–596. [PubMed] [Google Scholar]
- Ghersa P., Hooft van Huijsduijnen R., Whelan J., Cambet Y., Pescini R., DeLamarter J. F. Inhibition of E-selectin gene transcription through a cAMP-dependent protein kinase pathway. J Biol Chem. 1994 Nov 18;269(46):29129–29137. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F. Peritz' F test: basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput Biol Med. 1984;14(4):437–445. doi: 10.1016/0010-4825(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hourihan H., Allen T. D., Ager A. Lymphocyte migration across high endothelium is associated with increases in alpha 4 beta 1 integrin (VLA-4) affinity. J Cell Sci. 1993 Apr;104(Pt 4):1049–1059. doi: 10.1242/jcs.104.4.1049. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995 Jan 27;80(2):225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Johnson A., Phelps D. T., Ferro T. J. Tumor necrosis factor-alpha decreases pulmonary artery endothelial nitrovasodilator via protein kinase C. Am J Physiol. 1994 Sep;267(3 Pt 1):L318–L325. doi: 10.1152/ajplung.1994.267.3.L318. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S. S., Rangnekar V. V., Sells S. F., Rangnekar V. M. Interleukin-1-inducible expression of gro-beta via NF-kappa B activation is dependent upon tyrosine kinase signaling. J Biol Chem. 1993 Aug 25;268(24):18018–18029. [PubMed] [Google Scholar]
- Katabami T., Shimizu M., Okano K., Yano Y., Nemoto K., Ogura M., Tsukamoto T., Suzuki S., Ohira K., Yamada Y. Intracellular signal transduction for interleukin-1 beta-induced endothelin production in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1992 Oct 30;188(2):565–570. doi: 10.1016/0006-291x(92)91093-6. [DOI] [PubMed] [Google Scholar]
- Koroma B. M., de Juan E., Jr Phosphotyrosine inhibition and control of vascular endothelial cell proliferation by genistein. Biochem Pharmacol. 1994 Aug 17;48(4):809–818. doi: 10.1016/0006-2952(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W., Hakkert B. C., Hart M. H., Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol. 1992 May;117(3):565–572. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb P., Haslam J., Kessler L., Seidel H. M., Stein R. B., Rosen J. Rapid activation of the interferon-gamma signal transduction pathway by inhibitors of tyrosine phosphatases. J Interferon Res. 1994 Dec;14(6):365–373. doi: 10.1089/jir.1994.14.365. [DOI] [PubMed] [Google Scholar]
- Lane T. A., Lamkin G. E., Wancewicz E. Modulation of endothelial cell expression of intercellular adhesion molecule 1 by protein kinase C activation. Biochem Biophys Res Commun. 1989 Jun 30;161(3):945–952. doi: 10.1016/0006-291x(89)91334-x. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Neutrophils roll on E-selectin. J Immunol. 1993 Dec 1;151(11):6338–6346. [PubMed] [Google Scholar]
- Lee W. Y., Butler A. P., Locniskar M. F., Fischer S. M. Signal transduction pathway(s) involved in phorbol ester and autocrine induction of interleukin-1 alpha mRNA in murine keratinocytes. J Biol Chem. 1994 Jul 8;269(27):17971–17980. [PubMed] [Google Scholar]
- Leeuwenberg J. F., von Asmuth E. J., Jeunhomme T. M., Buurman W. A. IFN-gamma regulates the expression of the adhesion molecule ELAM-1 and IL-6 production by human endothelial cells in vitro. J Immunol. 1990 Oct 1;145(7):2110–2114. [PubMed] [Google Scholar]
- Ley K., Bullard D. C., Arbonés M. L., Bosse R., Vestweber D., Tedder T. F., Beaudet A. L. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995 Feb 1;181(2):669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look D. C., Pelletier M. R., Holtzman M. J. Selective interaction of a subset of interferon-gamma response element-binding proteins with the intercellular adhesion molecule-1 (ICAM-1) gene promoter controls the pattern of expression on epithelial cells. J Biol Chem. 1994 Mar 25;269(12):8952–8958. [PubMed] [Google Scholar]
- Magnuson D. K., Maier R. V., Pohlman T. H. Protein kinase C: a potential pathway of endothelial cell activation by endotoxin, tumor necrosis factor, and interleukin-1. Surgery. 1989 Aug;106(2):216–223. [PubMed] [Google Scholar]
- Marczin N., Papapetropoulos A., Catravas J. D. Tyrosine kinase inhibitors suppress endotoxin- and IL-1 beta-induced NO synthesis in aortic smooth muscle cells. Am J Physiol. 1993 Sep;265(3 Pt 2):H1014–H1018. doi: 10.1152/ajpheart.1993.265.3.H1014. [DOI] [PubMed] [Google Scholar]
- Mattila P., Majuri M. L., Mattila P. S., Renkonen R. TNF alpha-induced expression of endothelial adhesion molecules, ICAM-1 and VCAM-1, is linked to protein kinase C activation. Scand J Immunol. 1992 Aug;36(2):159–165. doi: 10.1111/j.1365-3083.1992.tb03087.x. [DOI] [PubMed] [Google Scholar]
- McGregor P. E., Agrawal D. K., Edwards J. D. Attenuation of human leukocyte adherence to endothelial cell monolayers by tyrosine kinase inhibitors. Biochem Biophys Res Commun. 1994 Jan 14;198(1):359–365. doi: 10.1006/bbrc.1994.1050. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Myers C. L., Wertheimer S. J., Schembri-King J., Parks T., Wallace R. W. Induction of ICAM-1 by TNF-alpha, IL-1 beta, and LPS in human endothelial cells after downregulation of PKC. Am J Physiol. 1992 Oct;263(4 Pt 1):C767–C772. doi: 10.1152/ajpcell.1992.263.4.C767. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991 Nov 1;147(9):2913–2921. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Palmer-Crocker R. L., Pober J. S. IL-4 induction of VCAM-1 on endothelial cells involves activation of a protein tyrosine kinase. J Immunol. 1995 Mar 15;154(6):2838–2845. [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Slowik M. R., De Luca L. G., Ritchie A. J. Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J Immunol. 1993 Jun 1;150(11):5114–5123. [PubMed] [Google Scholar]
- Ritchie A. J., Johnson D. R., Ewenstein B. M., Pober J. S. Tumor necrosis factor induction of endothelial cell surface antigens is independent of protein kinase C activation or inactivation. Studies with phorbol myristate acetate and staurosporine. J Immunol. 1991 May 1;146(9):3056–3062. [PubMed] [Google Scholar]
- Schütze S., Machleidt T., Krönke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J Leukoc Biol. 1994 Nov;56(5):533–541. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- Schütze S., Nottrott S., Pfizenmaier K., Krönke M. Tumor necrosis factor signal transduction. Cell-type-specific activation and translocation of protein kinase C. J Immunol. 1990 Apr 1;144(7):2604–2608. [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H., Hubscher S., Hirano H., Siebenlist U., Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993 Jan 7;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Tiisala S., Majuri M. L., Carpén O., Renkonen R. Genistein enhances the ICAM-mediated adhesion by inducing the expression of ICAM-1 and its counter-receptors. Biochem Biophys Res Commun. 1994 Aug 30;203(1):443–449. doi: 10.1006/bbrc.1994.2202. [DOI] [PubMed] [Google Scholar]
- Turunen J. P., Mattila P., Renkonen R. cAMP mediates IL-1-induced lymphocyte penetration through endothelial monolayers. J Immunol. 1990 Dec 15;145(12):4192–4197. [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H., Murakami Y., Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989 Sep 15;163(2):803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Umezawa K., Hori T., Tajima H., Imoto M., Isshiki K., Takeuchi T. Inhibition of epidermal growth factor-induced DNA synthesis by tyrosine kinase inhibitors. FEBS Lett. 1990 Jan 29;260(2):198–200. doi: 10.1016/0014-5793(90)80102-o. [DOI] [PubMed] [Google Scholar]
- Weber C., Negrescu E., Erl W., Pietsch A., Frankenberger M., Ziegler-Heitbrock H. W., Siess W., Weber P. C. Inhibitors of protein tyrosine kinase suppress TNF-stimulated induction of endothelial cell adhesion molecules. J Immunol. 1995 Jul 1;155(1):445–451. [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M., Mehra M., Prescott S. M. Endothelial cell-associated platelet-activating factor: a novel mechanism for signaling intercellular adhesion. J Cell Biol. 1990 Feb;110(2):529–540. doi: 10.1083/jcb.110.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Vermeer M., Koolwijk P., Grimbergen J., Kooistra T. Genistein reduces tumor necrosis factor alpha-induced plasminogen activator inhibitor-1 transcription but not urokinase expression in human endothelial cells. Blood. 1994 Nov 1;84(9):2984–2991. [PubMed] [Google Scholar]


