Abstract
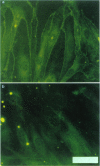
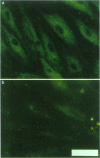
1. Morphine-6-glucuronide is one of the major metabolites of morphine. The potent analgesic action of this compound together with its potential lower apparent toxicity in man, when compared with morphine, indicated its clinical importance. 2. Primary cultures of porcine brain capillary endothelial cells were used to study brain penetration of morphine-6-glucuronide. Biochemical characterization of the cell cultures revealed a marked enrichment in enzymatic activity of alkaline phosphatase (56 fold) and angiotensin converting enzyme (230 fold) as compared to whole brain tissue. By immunostaining the presence of vimentin, factor VIII, the tight junction associated protein ZO-1, and P-glycoprotein was shown. Functional characterization revealed that the carrier system responsible for transport of neutral amino acids was intact. 3. Uptake and transport of morphine-6-glucuronide was marginal and in the range of the extracellular marker sucrose. However, uptake of morphine-6-glucuronide was enhanced significantly (P < 0.0001) in presence of the inhibitors of P-glycoprotein, verapamil or vincristine. The finding that morphine-6-glucuronide may serve as a substrate for P-glycoprotein was confirmed in multidrug-resistant P388 tumour cells. 4. We conclude that penetration of the blood-brain barrier by morphine-6-glucuronide may depend on the expression of the product of the multidrug-resistance (MDR) gene in brain capillary endothelial cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaulieu E., Demeule M., Pouliot J. F., Averill-Bates D. A., Murphy G. F., Béliveau R. P-glycoprotein of blood brain barrier: cross-reactivity of Mab C219 with a 190 kDa protein in bovine and rat isolated brain capillaries. Biochim Biophys Acta. 1995 Jan 26;1233(1):27–32. doi: 10.1016/0005-2736(94)00239-l. [DOI] [PubMed] [Google Scholar]
- Boesch D., Gavériaux C., Jachez B., Pourtier-Manzanedo A., Bollinger P., Loor F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991 Aug 15;51(16):4226–4233. [PubMed] [Google Scholar]
- Callaghan R., Riordan J. R. Synthetic and natural opiates interact with P-glycoprotein in multidrug-resistant cells. J Biol Chem. 1993 Jul 25;268(21):16059–16064. [PubMed] [Google Scholar]
- Carrupt P. A., Testa B., Bechalany A., el Tayar N., Descas P., Perrissoud D. Morphine 6-glucuronide and morphine 3-glucuronide as molecular chameleons with unexpected lipophilicity. J Med Chem. 1991 Apr;34(4):1272–1275. doi: 10.1021/jm00108a005. [DOI] [PubMed] [Google Scholar]
- Chesné C., Dehouck M. P., Jolliet-Riant P., Brée F., Tillement J. P., Dehouck B., Fruchart J. C., Cecchelli R. Drug transfer across the blood-brain barrier: comparison of in vitro and in vivo models. Adv Exp Med Biol. 1993;331:113–115. doi: 10.1007/978-1-4615-2920-0_18. [DOI] [PubMed] [Google Scholar]
- Dehouck M. P., Jolliet-Riant P., Brée F., Fruchart J. C., Cecchelli R., Tillement J. P. Drug transfer across the blood-brain barrier: correlation between in vitro and in vivo models. J Neurochem. 1992 May;58(5):1790–1797. doi: 10.1111/j.1471-4159.1992.tb10055.x. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Friedland J., Silverstein E. A sensitive fluorimetric assay for serum angiotensin-converting enzyme. Am J Clin Pathol. 1976 Aug;66(2):416–424. doi: 10.1093/ajcp/66.2.416. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Wolinsky J. S., Csejtey J., Diamond I. Isolation of metabolically active capillaries from rat brain. J Neurochem. 1975 Nov;25(5):715–717. doi: 10.1111/j.1471-4159.1975.tb04395.x. [DOI] [PubMed] [Google Scholar]
- Gosland M., Tsuboi C., Hoffman T., Goodin S., Vore M. 17 beta-estradiol glucuronide: an inducer of cholestasis and a physiological substrate for the multidrug resistance transporter. Cancer Res. 1993 Nov 15;53(22):5382–5385. [PubMed] [Google Scholar]
- Hucks D., Thompson P. I., McLoughlin L., Joel S. P., Patel N., Grossman A., Rees L. H., Slevin M. L. Explanation at the opioid receptor level for differing toxicity of morphine and morphine 6-glucuronide. Br J Cancer. 1992 Jan;65(1):122–126. doi: 10.1038/bjc.1992.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. C., Lantos P. L. Uptake of leucine and alanine by cultured cerebral capillary endothelial cells. Brain Res. 1989 Feb 20;480(1-2):126–132. doi: 10.1016/0006-8993(89)91575-8. [DOI] [PubMed] [Google Scholar]
- Huwyler J., Rufer S., Küsters E., Drewe J. Rapid and highly automated determination of morphine and morphine glucuronides in plasma by on-line solid-phase extraction and column liquid chromatography. J Chromatogr B Biomed Appl. 1995 Dec 1;674(1):57–63. doi: 10.1016/0378-4347(95)00295-7. [DOI] [PubMed] [Google Scholar]
- McQuay H. J., Carroll D., Faura C. C., Gavaghan D. J., Hand C. W., Moore R. A. Oral morphine in cancer pain: influences on morphine and metabolite concentration. Clin Pharmacol Ther. 1990 Sep;48(3):236–244. doi: 10.1038/clpt.1990.145. [DOI] [PubMed] [Google Scholar]
- Murphey L. J., Olsen G. D. Diffusion of morphine-6-beta-D-glucuronide into the neonatal guinea pig brain during drug-induced respiratory depression. J Pharmacol Exp Ther. 1994 Oct;271(1):118–124. [PubMed] [Google Scholar]
- Osborne R., Thompson P., Joel S., Trew D., Patel N., Slevin M. The analgesic activity of morphine-6-glucuronide. Br J Clin Pharmacol. 1992 Aug;34(2):130–138. doi: 10.1111/j.1365-2125.1992.tb04121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta. 1975 Aug 5;401(1):128–136. doi: 10.1016/0005-2736(75)90347-8. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Triguero D., Yang J., Cancilla P. A. Comparison of in vitro and in vivo models of drug transcytosis through the blood-brain barrier. J Pharmacol Exp Ther. 1990 May;253(2):884–891. [PubMed] [Google Scholar]
- Pasternak G. W., Bodnar R. J., Clark J. A., Inturrisi C. E. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987 Dec 28;41(26):2845–2849. doi: 10.1016/0024-3205(87)90431-0. [DOI] [PubMed] [Google Scholar]
- Paul D., Standifer K. M., Inturrisi C. E., Pasternak G. W. Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J Pharmacol Exp Ther. 1989 Nov;251(2):477–483. [PubMed] [Google Scholar]
- Polt R., Porreca F., Szabò L. Z., Bilsky E. J., Davis P., Abbruscato T. J., Davis T. P., Harvath R., Yamamura H. I., Hruby V. J. Glycopeptide enkephalin analogues produce analgesia in mice: evidence for penetration of the blood-brain barrier. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7114–7118. doi: 10.1073/pnas.91.15.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelstra E. C., Westerhoff H. V., Pinedo H. M., Dekker H., Lankelma J. The multidrug-resistance-reverser verapamil interferes with cellular P-glycoprotein-mediated pumping of daunorubicin as a non-competing substrate. Eur J Biochem. 1994 Apr 1;221(1):363–373. doi: 10.1111/j.1432-1033.1994.tb18748.x. [DOI] [PubMed] [Google Scholar]
- Sugawara I., Hamada H., Tsuruo T., Mori S. Specialized localization of P-glycoprotein recognized by MRK 16 monoclonal antibody in endothelial cells of the brain and the spinal cord. Jpn J Cancer Res. 1990 Aug;81(8):727–730. doi: 10.1111/j.1349-7006.1990.tb02636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T., Naito M., Oh-hara T., Sugawara I., Tsuruo T. Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem. 1992 Oct 5;267(28):20383–20391. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P. I., Joel S. P., John L., Wedzicha J. A., Maclean M., Slevin M. L. Respiratory depression following morphine and morphine-6-glucuronide in normal subjects. Br J Clin Pharmacol. 1995 Aug;40(2):145–152. [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Terasaki T., Takabatake Y., Tenda Y., Tamai I., Yamashima T., Moritani S., Tsuruo T., Yamashita J. P-glycoprotein as the drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci. 1992;51(18):1427–1437. doi: 10.1016/0024-3205(92)90537-y. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Cyclosporins as drug resistance modifiers. Biochem Pharmacol. 1992 Jan 9;43(1):109–117. doi: 10.1016/0006-2952(92)90668-9. [DOI] [PubMed] [Google Scholar]
- Van Crugten J. T., Sallustio B. C., Nation R. L., Somogyi A. A. Renal tubular transport of morphine, morphine-6-glucuronide, and morphine-3-glucuronide in the isolated perfused rat kidney. Drug Metab Dispos. 1991 Nov-Dec;19(6):1087–1092. [PubMed] [Google Scholar]
- Yoshimura H., Ida S., Oguri K., Tsukamoto H. Biochemical basis for analgesic activity of morphine-6-glucuronide. I. Penetration of morphine-6-glucuronide in the brain of rats. Biochem Pharmacol. 1973 Jun 15;22(12):1423–1430. doi: 10.1016/0006-2952(73)90320-1. [DOI] [PubMed] [Google Scholar]