Abstract
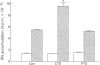
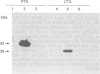
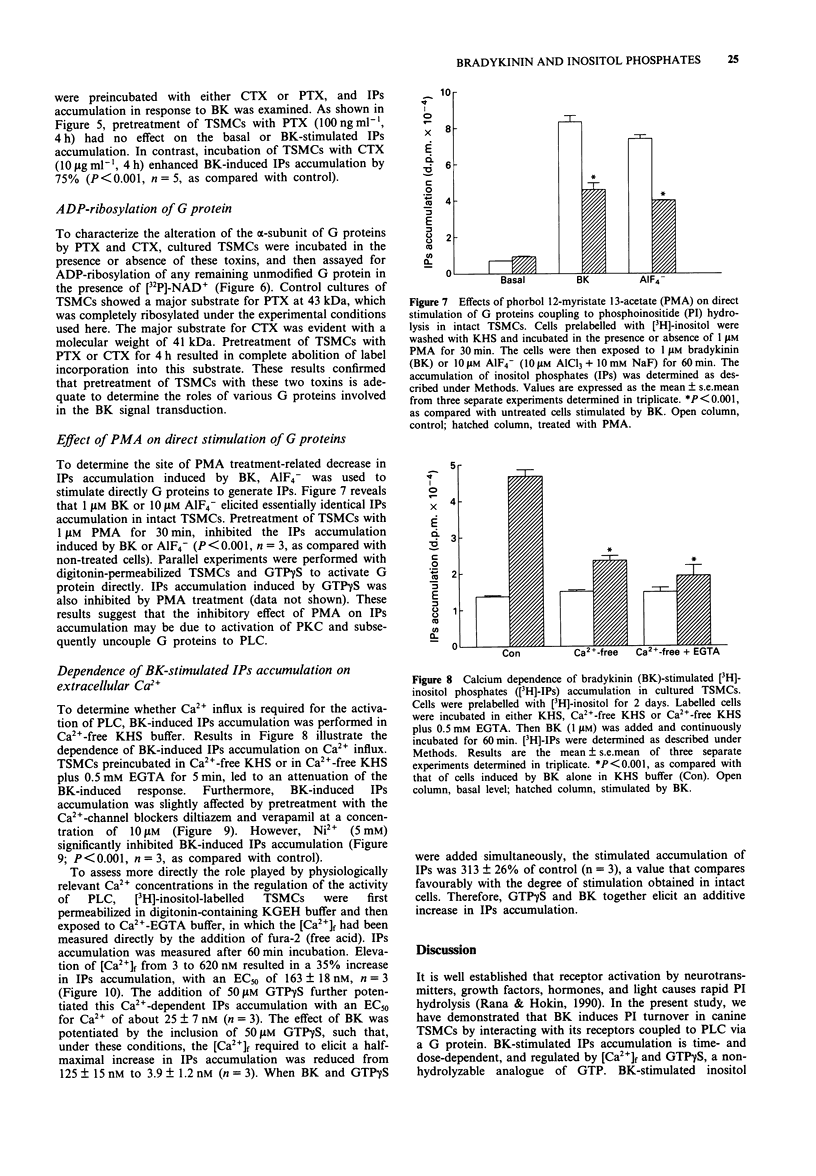
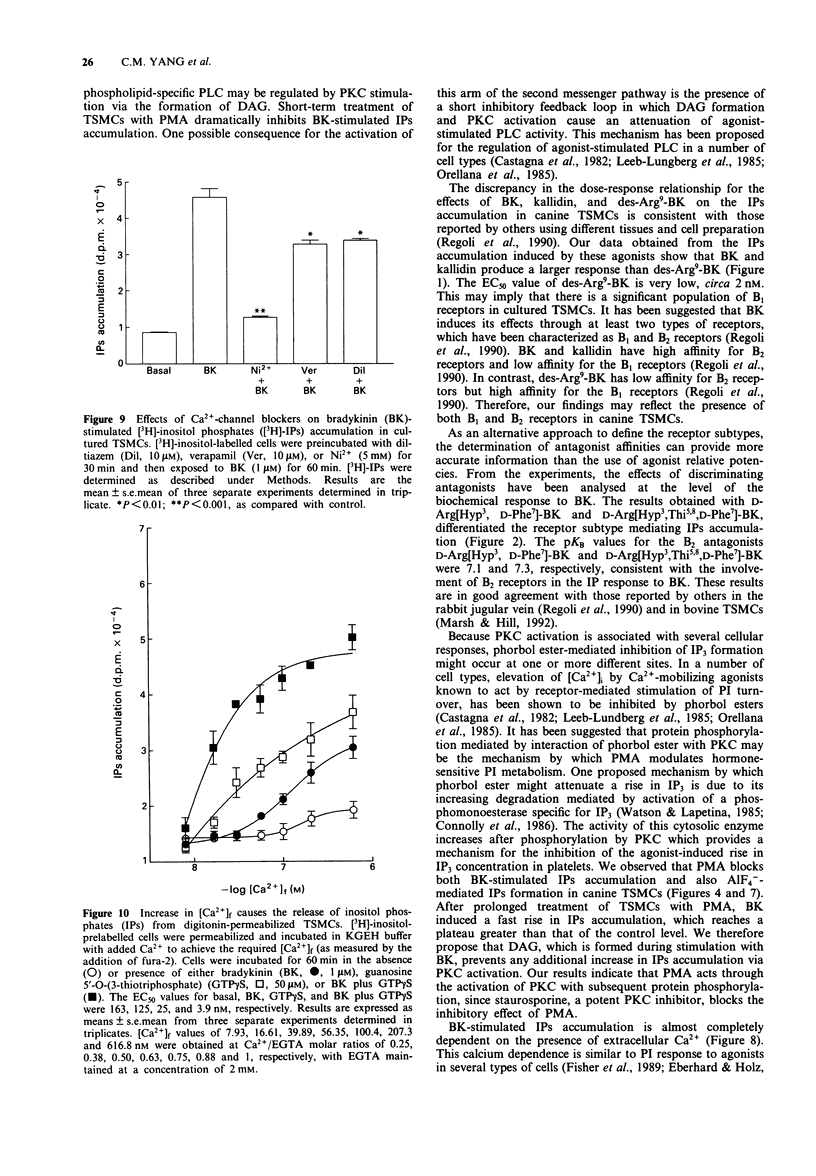
1. Stimulation of bradykinin (BK) receptors coupled to phosphoinositide (PI) hydrolysis was investigated in canine cultured tracheal smooth muscle cells (TSMCs). BK, kallidin, and des-Arg9-BK, stimulated [3H]-inositol phosphates (IPs) accumulation in a dose-dependent manner with half-maximal responses (EC50) at 20 +/- 5, 13 +/- 4, and 2.3 +/- 0.7 nM, (n = 5), respectively. 2. D-Arg[Hyp3, D-Phe7]-BK and D-Arg[Hyp3, Thi5,8, D-Phe7]-BK, B2 receptor antagonists, were equipotent in blocking the BK-induced IPs accumulation with pKB = 7.1 and 7.3, respectively. 3. Short-term exposure of TSMCs to phorbol 12-myristate 13-acetate (PMA, 1 microM) attenuated BK-stimulated IPs accumulation. The concentrations of PMA that gave half-maximal and maximal inhibition of BK-induced IPs accumulation were 15 +/- 4 nM and 1 microM, n = 3, respectively. The inhibitory effect of PMA on BK-induced response was reversed by staurosporine, a protein kinase C (PKC) inhibitor, suggesting that the inhibitory effect of PMA was mediated through the activation of PKC. 4. Prolonged incubation of TSMCs with PMA for 24 h, resulted in a recovery of receptor responsiveness which may be due to down-regulation of PKC. The inactive phorbol ester, 4 alpha-phorbol 12, 13-didecanoate at 1 microM, did not inhibit this response. 5. The site of this inhibition was further investigated by examining the effect of PMA on AlF(4-)-induced IPs accumulation in canine TSMCs. AlF(4-)-stimulated IPs accumulation was inhibited by PMA treatment, suggesting that the G protein(s) can be directly activated by AlF4-, which is uncoupled from phospholipase C by PMA treatment. 6. Incubation of TSMCs in the absence of external Ca2+ or upon removal of Ca2+ by addition of EGTA, caused a decrease in IPs accumulation without changing the basal levels. Addition of Ca2+ (3-620 nM) to digitonin-permeabilized TSMCs stimulated IPs accumulation was obtained by inclusion of either guanosine 5'-O-(3-thiotriphosphate) (GTP gamma S) or BK.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aub D. L., Frey E. A., Sekura R. D., Cote T. E. Coupling of the thyrotropin-releasing hormone receptor to phospholipase C by a GTP-binding protein distinct from the inhibitory or stimulatory GTP-binding protein. J Biol Chem. 1986 Jul 15;261(20):9333–9340. [PubMed] [Google Scholar]
- Barnes P. J. Modulation of neurotransmission in airways. Physiol Rev. 1992 Jul;72(3):699–729. doi: 10.1152/physrev.1992.72.3.699. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Braas K. M., Manning D. C., Perry D. C., Snyder S. H. Bradykinin analogues: differential agonist and antagonist activities suggesting multiple receptors. Br J Pharmacol. 1988 May;94(1):3–5. doi: 10.1111/j.1476-5381.1988.tb11492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Guanine nucleotide regulation of phospholipase C activity in permeabilized rabbit neutrophils. Inhibition by pertussis toxin and sensitization to submicromolar calcium concentrations. Biochem J. 1986 Oct 1;239(1):97–102. doi: 10.1042/bj2390097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Christiansen S. C., Proud D., Cochrane C. G. Detection of tissue kallikrein in the bronchoalveolar lavage fluid of asthmatic subjects. J Clin Invest. 1987 Jan;79(1):188–197. doi: 10.1172/JCI112782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Lawing W. J., Jr, Majerus P. W. Protein kinase C phosphorylates human platelet inositol trisphosphate 5'-phosphomonoesterase, increasing the phosphatase activity. Cell. 1986 Sep 12;46(6):951–958. doi: 10.1016/0092-8674(86)90077-2. [DOI] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Cholinergic stimulation of inositol phosphate formation in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic mechanisms. J Neurochem. 1987 Nov;49(5):1634–1643. doi: 10.1111/j.1471-4159.1987.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Regulation of the formation of inositol phosphates by calcium, guanine nucleotides and ATP in digitonin-permeabilized bovine adrenal chromaffin cells. Biochem J. 1991 Oct 15;279(Pt 2):447–453. doi: 10.1042/bj2790447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etscheid B. G., Villereal M. L. Coupling of bradykinin receptors to phospholipase C in cultured fibroblasts is mediated by a G-protein. J Cell Physiol. 1989 Aug;140(2):264–271. doi: 10.1002/jcp.1041400211. [DOI] [PubMed] [Google Scholar]
- Evans T., Hepler J. R., Masters S. B., Brown J. H., Harden T. K. Guanine nucleotide regulation of agonist binding to muscarinic cholinergic receptors. Relation to efficacy of agonists for stimulation of phosphoinositide breakdown and Ca2+ mobilization. Biochem J. 1985 Dec 15;232(3):751–757. doi: 10.1042/bj2320751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Domask L. M., Roland R. M. Muscarinic receptor regulation of cytoplasmic Ca2+ concentrations in human SK-N-SH neuroblastoma cells: Ca2+ requirements for phospholipase C activation. Mol Pharmacol. 1989 Feb;35(2):195–204. [PubMed] [Google Scholar]
- Galron R., Bdolah A., Kloog Y., Sokolovsky M. Endothelin/sarafotoxin receptor induced phosphoinositide turnover: effects of pertussis and cholera toxins and of phorbol ester. Biochem Biophys Res Commun. 1990 Sep 28;171(3):949–954. doi: 10.1016/0006-291x(90)90776-j. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski S., Smrcka A., Nowak L., Wu D. G., Simon M., Sternweis P. C. Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991 Oct 25;266(30):20519–20524. [PubMed] [Google Scholar]
- Hepler J. R., Hughes A. R., Harden T. K. Evidence that muscarinic cholinergic receptors selectively interact with either the cyclic AMP or the inositol phosphate second-messenger response systems. Biochem J. 1987 Nov 1;247(3):793–796. doi: 10.1042/bj2470793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H., Streaty R. A., Klee W., Nirenberg M. Bradykinin-activated transmembrane signals are coupled via No or Ni to production of inositol 1,4,5-trisphosphate, a second messenger in NG108-15 neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):942–946. doi: 10.1073/pnas.83.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. G., Goldstein D., Brown J. H. Guanine nucleotide-dependent inositol trisphosphate formation in chick heart cells. Circ Res. 1988 Feb;62(2):299–305. doi: 10.1161/01.res.62.2.299. [DOI] [PubMed] [Google Scholar]
- Kurose H., Ui M. Functional uncoupling of muscarinic receptors from adenylate cyclase in rat cardiac membranes by the active component of islet-activating protein, pertussis toxin. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(4-5):305–318. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann C., Schnittler M., Stewart J. M., Reissmann S. Antagonist binding reveals two heterogenous B2 bradykinin receptors in rat myometrial membranes. Eur J Pharmacol. 1991 Jul 9;199(3):363–365. doi: 10.1016/0014-2999(91)90501-g. [DOI] [PubMed] [Google Scholar]
- Manning D. C., Vavrek R., Stewart J. M., Snyder S. H. Two bradykinin binding sites with picomolar affinities. J Pharmacol Exp Ther. 1986 May;237(2):504–512. [PubMed] [Google Scholar]
- Marsh K. A., Hill S. J. Bradykinin B2 receptor-mediated phosphoinositide hydrolysis in bovine cultured tracheal smooth muscle cells. Br J Pharmacol. 1992 Oct;107(2):443–447. doi: 10.1111/j.1476-5381.1992.tb12765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. W., Evans T., Harden T. K. Further evidence that muscarinic cholinergic receptors of 1321N1 astrocytoma cells couple to a guanine nucleotide regulatory protein that is not Ni. Biochem J. 1985 Jul 15;229(2):539–544. doi: 10.1042/bj2290539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T., Ui M. Phosphatidic acid may stimulate membrane receptors mediating adenylate cyclase inhibition and phospholipid breakdown in 3T3 fibroblasts. J Biol Chem. 1987 Apr 25;262(12):5522–5529. [PubMed] [Google Scholar]
- Murray R. K., Bennett C. F., Fluharty S. J., Kotlikoff M. I. Mechanism of phorbol ester inhibition of histamine-induced IP3 formation in cultured airway smooth muscle. Am J Physiol. 1989 Oct;257(4 Pt 1):L209–L216. doi: 10.1152/ajplung.1989.257.4.L209. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Orellana S. A., Solski P. A., Brown J. H. Phorbol ester inhibits phosphoinositide hydrolysis and calcium mobilization in cultured astrocytoma cells. J Biol Chem. 1985 May 10;260(9):5236–5239. [PubMed] [Google Scholar]
- Osugi T., Imaizumi T., Mizushima A., Uchida S., Yoshida H. Role of a protein regulating guanine nucleotide binding in phosphoinositide breakdown and calcium mobilization by bradykinin in neuroblastoma X glioma hybrid NG108-15 cells: effects of pertussis toxin and cholera toxin on receptor-mediated signal transduction. Eur J Pharmacol. 1987 Jun 4;137(2-3):207–218. doi: 10.1016/0014-2999(87)90224-x. [DOI] [PubMed] [Google Scholar]
- Plevin R., Owen P. J. Multiple B2 kinin receptors in mammalian tissues. Trends Pharmacol Sci. 1988 Nov;9(11):387–389. doi: 10.1016/0165-6147(88)90059-4. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Regoli D., Rhaleb N. E., Dion S., Drapeau G. New selective bradykinin receptor antagonists and bradykinin B2 receptor characterization. Trends Pharmacol Sci. 1990 Apr;11(4):156–161. doi: 10.1016/0165-6147(90)90067-I. [DOI] [PubMed] [Google Scholar]
- Reiser G., Binmöller F. J., Donié F. Mechanisms for activation and subsequent removal of cytosolic Ca2+ in bradykinin-stimulated neuronal and glial cell lines. Exp Cell Res. 1990 Jan;186(1):47–53. doi: 10.1016/0014-4827(90)90208-r. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. M., Berry G. T., Yandrasitz J. R., Grunstein M. M. Maturational regulation of inositol 1,4,5-trisphosphate metabolism in rabbit airway smooth muscle. J Clin Invest. 1991 Dec;88(6):2032–2038. doi: 10.1172/JCI115531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Cox C. C., Snyderman R. Receptor-coupled activation of phosphoinositide-specific phospholipase C by an N protein. Science. 1986 Apr 4;232(4746):97–100. doi: 10.1126/science.3006254. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Smrcka A. V. Regulation of phospholipase C by G proteins. Trends Biochem Sci. 1992 Dec;17(12):502–506. doi: 10.1016/0968-0004(92)90340-f. [DOI] [PubMed] [Google Scholar]
- Trifilieff A., Haddad E. B., Landry Y., Gies J. P. Evidence for two high-affinity bradykinin binding sites in the guinea-pig lung. Eur J Pharmacol. 1991 Jun 19;207(2):129–134. doi: 10.1016/0922-4106(91)90087-x. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Lapetina E. G. 1,2-Diacylglycerol and phorbol ester inhibit agonist-induced formation of inositol phosphates in human platelets: possible implications for negative feedback regulation of inositol phospholipid hydrolysis. Proc Natl Acad Sci U S A. 1985 May;82(9):2623–2626. doi: 10.1073/pnas.82.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. M., Chou S. P., Sung T. C., Chien H. J. Regulation of functional muscarinic receptor expression in tracheal smooth muscle cells. Am J Physiol. 1991 Dec;261(6 Pt 1):C1123–C1129. doi: 10.1152/ajpcell.1991.261.6.C1123. [DOI] [PubMed] [Google Scholar]