Abstract
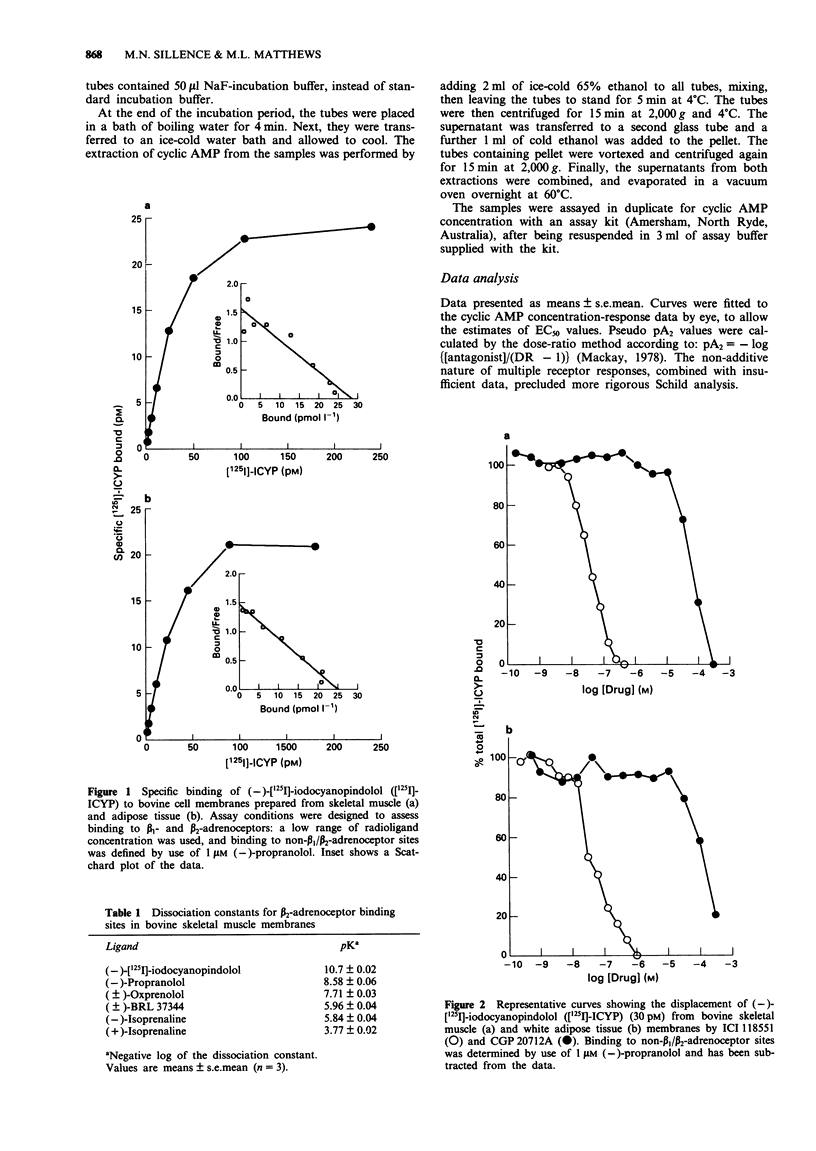
1. The radioligand [125I]-iodocyanopindolol ([125I]-ICYP) was used under standard ligand binding conditions, to detect beta 1- and beta 2-adrenoceptors in membrane preparations from bovine skeletal muscle and adipose tissue. High concentrations of [125I]-ICYP were also used, to identify an 'atypical' binding site in skeletal muscle. Finally, adenosine 3':5'-cyclic monophosphate (cyclic AMP) production was measured in the same membrane preparations, to determine the relationship between the beta-adrenoceptor sub-types present and the production of this second-messenger. 2. According to the results of radioligand binding studies, both skeletal muscle and adipose tissue membranes have beta 2-adrenoceptors, characterized by a high affinity for the beta 2-selective antagonist, ICI 118551 (pK 8.3 and 8.6 respectively); and a low affinity for the beta 1-selective antagonist CGP 20712A (pK 5.2 in both tissues). Antagonism of (-)-isoprenaline-stimulated cyclic AMP production by low concentrations of ICI 118551, yielded pseudo pA2 values in muscle and adipose tissue of 7.6 and 8.7 respectively, confirming that beta 2-adrenoceptors in these tissues are linked to the production of the second-messenger. 3. Although beta 1-adrenoceptors could not be detected in either skeletal muscle or adipose tissue membranes by use of ligand binding techniques, high pseudo pA2 values were obtained (8.0 and 8.2 respectively), when CGP 20712A was used to block the stimulation of cyclic AMP production by (-)-isoprenaline. This finding is consistent with the presence in both tissues of a population of beta 1-adrenoceptors which is small, but efficiently coupled to the second-messenger.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Ainsworth A. T., Cawthorne M. A., Piercy V., Sennitt M. V., Thody V. E., Wilson C., Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984 May 10;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- Arner P. Adrenergic receptor function in fat cells. Am J Clin Nutr. 1992 Jan;55(1 Suppl):228S–236S. doi: 10.1093/ajcn/55.1.228s. [DOI] [PubMed] [Google Scholar]
- Björgell P., Belfrage P. Characteristics of the lipolytic beta-adrenergic receptors in hamster adipocytes. Biochim Biophys Acta. 1982 Oct 14;713(1):80–85. doi: 10.1016/0005-2760(82)90169-2. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Leighton B., Wilson S., Thurlby P. L., Arch J. R. An investigation of the beta-adrenoceptor that mediates metabolic responses to the novel agonist BRL28410 in rat soleus muscle. Biochem Pharmacol. 1988 Mar 1;37(5):947–950. doi: 10.1016/0006-2952(88)90186-4. [DOI] [PubMed] [Google Scholar]
- Coutinho L. L., Bergen W. G., Merkel R. A., Smith C. K., 2nd Quantitative characterization of beta-adrenergic receptor subtypes in porcine adipocytes. Comp Biochem Physiol C. 1992 Apr;101(3):481–485. doi: 10.1016/0742-8413(92)90074-h. [DOI] [PubMed] [Google Scholar]
- Disatnik M. H., Sampson S. R., Shainberg A. Characterization of beta-adrenoceptors on rat skeletal muscle cells grown in vitro. Biochem Pharmacol. 1990 Sep 1;40(5):1043–1048. doi: 10.1016/0006-2952(90)90491-3. [DOI] [PubMed] [Google Scholar]
- Elfellah M. S., Reid J. L. Regulation of beta 1- and beta 2-adrenoceptors following chronic treatment with beta-adrenoceptor antagonists. Eur J Pharmacol. 1989 Nov 28;173(1):85–92. doi: 10.1016/0014-2999(89)90011-3. [DOI] [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Lasnier F., Blin N., Baude B., Nahmias C., Strosberg A. D., Pairault J. Atypical beta-adrenergic receptor in 3T3-F442A adipocytes. Pharmacological and molecular relationship with the human beta 3-adrenergic receptor. J Biol Chem. 1991 Oct 25;266(30):20329–20336. [PubMed] [Google Scholar]
- Granneman J. G. Effects of agonist exposure on the coupling of beta 1 and beta 3 adrenergic receptors to adenylyl cyclase in isolated adipocytes. J Pharmacol Exp Ther. 1992 May;261(2):638–642. [PubMed] [Google Scholar]
- Granneman J. G. Norepinephrine and BRL 37344 stimulate adenylate cyclase by different receptors in rat brown adipose tissue. J Pharmacol Exp Ther. 1990 Aug;254(2):508–513. [PubMed] [Google Scholar]
- Hollenga C., Brouwer F., Zaagsma J. Differences in functional cyclic AMP compartments mediating lipolysis by isoprenaline and BRL 37344 in four adipocyte types. Eur J Pharmacol. 1991 Aug 6;200(2-3):325–330. doi: 10.1016/0014-2999(91)90590-m. [DOI] [PubMed] [Google Scholar]
- Hollenga C., Brouwer F., Zaagsma J. Relationship between lipolysis and cyclic AMP generation mediated by atypical beta-adrenoceptors in rat adipocytes. Br J Pharmacol. 1991 Mar;102(3):577–580. doi: 10.1111/j.1476-5381.1991.tb12215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenga C., Zaagsma J. Direct evidence for the atypical nature of functional beta-adrenoceptors in rat adipocytes. Br J Pharmacol. 1989 Dec;98(4):1420–1424. doi: 10.1111/j.1476-5381.1989.tb12692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D., Engel G., Kalkman H. O. Characterization of the 5-HT1B recognition site in rat brain: binding studies with (-)[125I]iodocyanopindolol. Eur J Pharmacol. 1985 Nov 26;118(1-2):1–12. doi: 10.1016/0014-2999(85)90657-0. [DOI] [PubMed] [Google Scholar]
- IJzerman A. P., Bultsma T., Timmerman H., Zaagsma J. The relation between ionization and affinity of beta-adrenoceptor ligands. Naunyn Schmiedebergs Arch Pharmacol. 1984 Oct;327(4):293–298. doi: 10.1007/BF00506239. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Sainz R. D., Molenaar P., Summers R. J. Characterization of beta 1- and beta 2-adrenoceptors in rat skeletal muscles. Biochem Pharmacol. 1991 Oct 9;42(9):1783–1789. doi: 10.1016/0006-2952(91)90516-8. [DOI] [PubMed] [Google Scholar]
- Langin D., Portillo M. P., Saulnier-Blache J. S., Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol. 1991 Jul 9;199(3):291–301. doi: 10.1016/0014-2999(91)90492-9. [DOI] [PubMed] [Google Scholar]
- Liggett S. B., Shah S. D., Cryer P. E. Characterization of beta-adrenergic receptors of human skeletal muscle obtained by needle biopsy. Am J Physiol. 1988 Jun;254(6 Pt 1):E795–E798. doi: 10.1152/ajpendo.1988.254.6.E795. [DOI] [PubMed] [Google Scholar]
- MacKay D. How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated? J Pharm Pharmacol. 1978 May;30(5):312–313. doi: 10.1111/j.2042-7158.1978.tb13237.x. [DOI] [PubMed] [Google Scholar]
- Mauriège P., De Pergola G., Berlan M., Lafontan M. Human fat cell beta-adrenergic receptors: beta-agonist-dependent lipolytic responses and characterization of beta-adrenergic binding sites on human fat cell membranes with highly selective beta 1-antagonists. J Lipid Res. 1988 May;29(5):587–601. [PubMed] [Google Scholar]
- Molenaar P., Roberts S. J., Kim Y. S., Pak H. S., Sainz R. D., Summers R. J. Localization and characterization of two propranolol resistant (-) [125I]cyanopindolol binding sites in rat skeletal muscle. Eur J Pharmacol. 1991 Dec 17;209(3):257–262. doi: 10.1016/0014-2999(91)90179-t. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nahmias C., Blin N., Elalouf J. M., Mattei M. G., Strosberg A. D., Emorine L. J. Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991 Dec;10(12):3721–3727. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker B., Kramer K., Stroes J. W., Vlug J., Krielaart M., Zaagsma J. High affinity non-beta-adrenoceptor binding of beta-adrenergic ligands. Eur J Pharmacol. 1985 Apr 23;111(1):31–36. doi: 10.1016/0014-2999(85)90110-4. [DOI] [PubMed] [Google Scholar]
- Rigby P. J., Passarelli M. C., Self G. J., Preuss J. M., Goldie R. G. Ascorbic acid-induced binding of [125I]-iodocyanopindolol to non-beta-adrenoceptor sites in guinea-pig trachea. Biochem Pharmacol. 1988 Apr 1;37(7):1421–1424. doi: 10.1016/0006-2952(88)90804-0. [DOI] [PubMed] [Google Scholar]
- Roberts S. J., Molenaar P., Summers R. J. Characterization of propranolol-resistant (-)-[125I]-cyanopindolol binding sites in rat soleus muscle. Br J Pharmacol. 1993 Jun;109(2):344–352. doi: 10.1111/j.1476-5381.1993.tb13576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence M. N., Moore N. G., Pegg G. G., Lindsay D. B. Ligand binding properties of putative beta 3-adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br J Pharmacol. 1993 Aug;109(4):1157–1163. doi: 10.1111/j.1476-5381.1993.tb13743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence M. N., Pegg G. G., Lindsay D. B. Affinity of clenbuterol analogues for beta 2-adrenoceptors in bovine skeletal muscle and the effect of these compounds on urinary nitrogen excretion in female rats. Naunyn Schmiedebergs Arch Pharmacol. 1991 Oct;344(4):442–448. doi: 10.1007/BF00172584. [DOI] [PubMed] [Google Scholar]
- Wilson C., Wilson S., Piercy V., Sennitt M. V., Arch J. R. The rat lipolytic beta-adrenoceptor: studies using novel beta-adrenoceptor agonists. Eur J Pharmacol. 1984 May 4;100(3-4):309–319. doi: 10.1016/0014-2999(84)90007-4. [DOI] [PubMed] [Google Scholar]


