Abstract
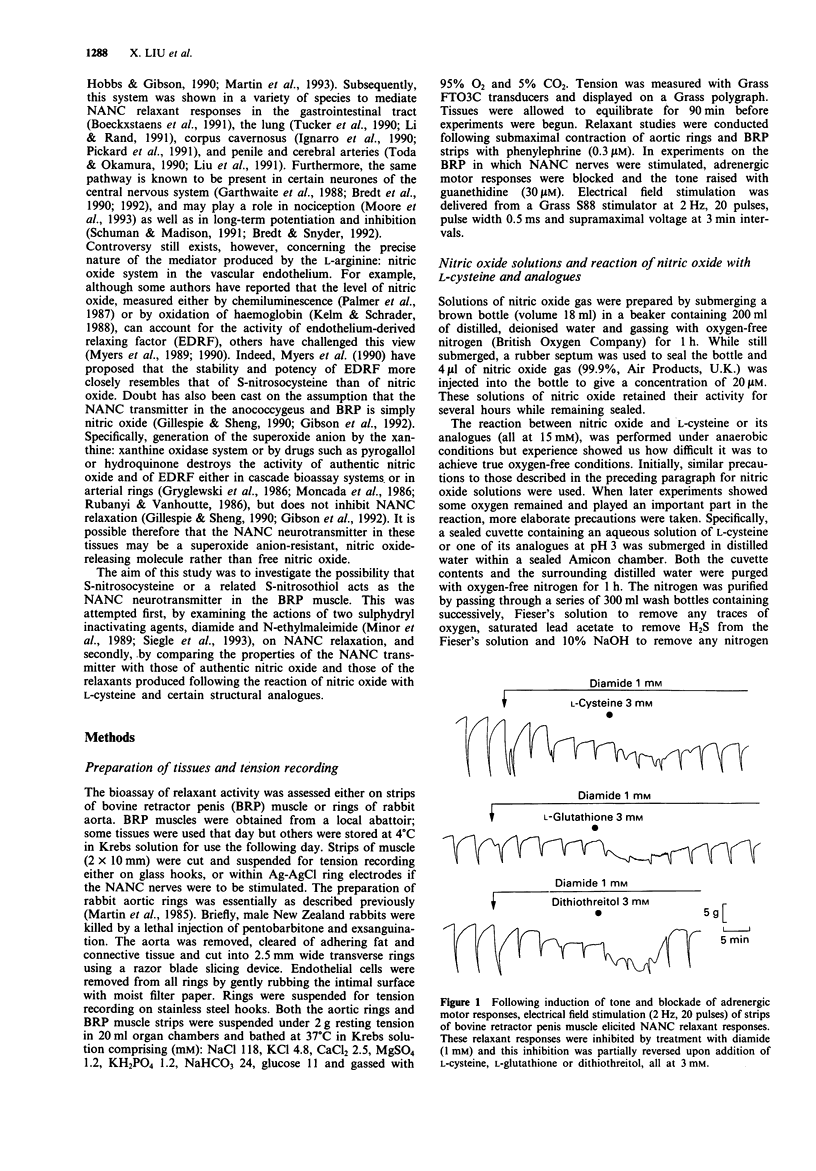
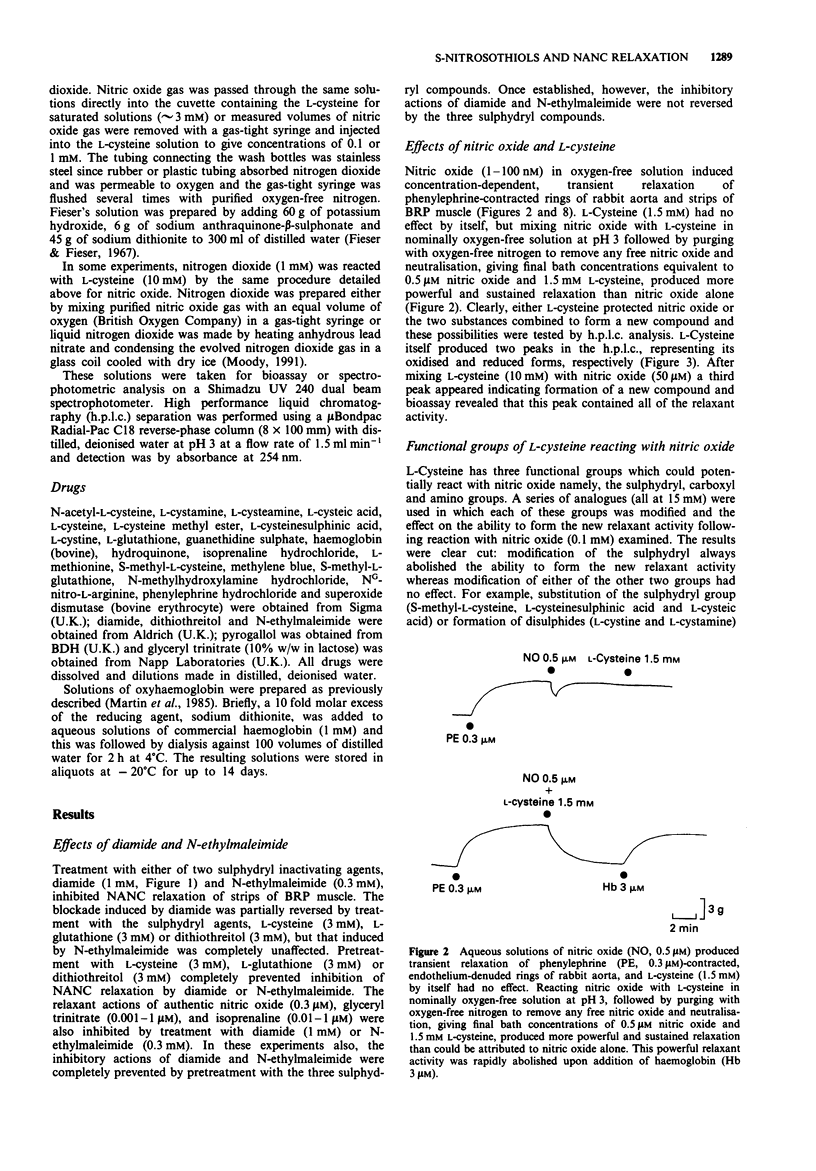
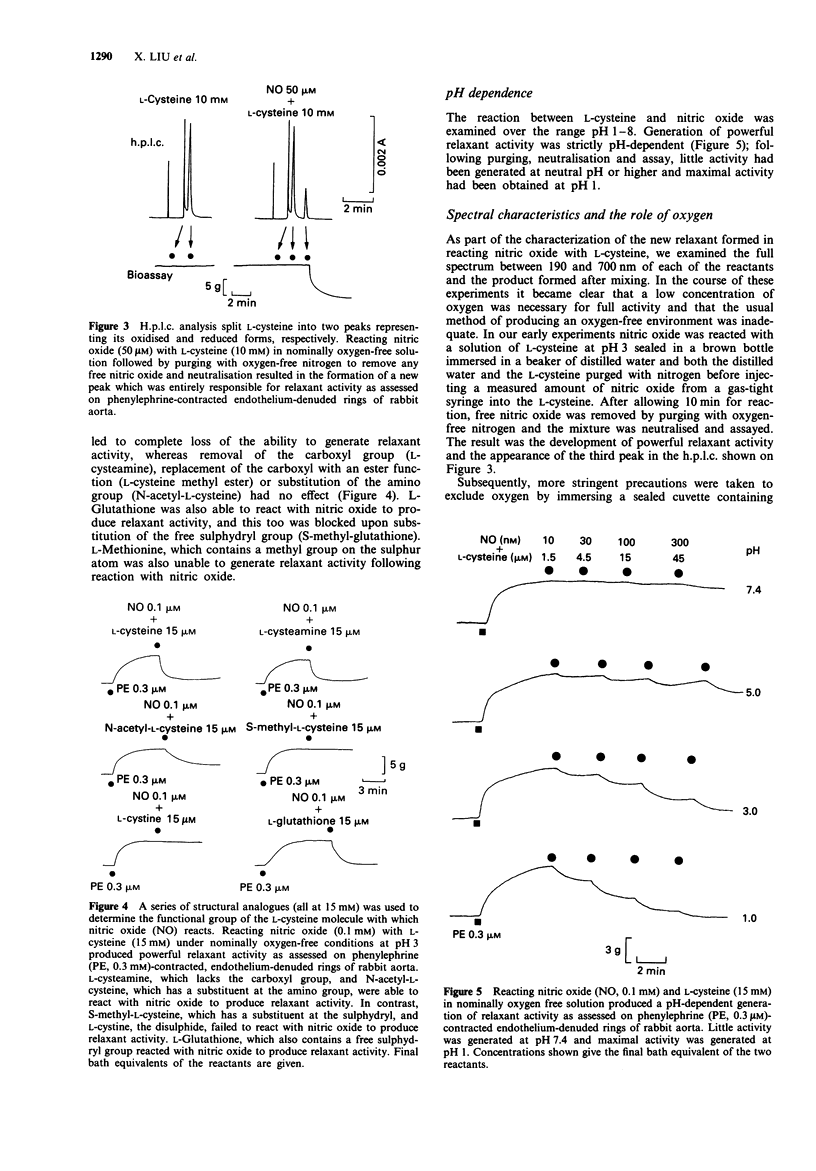
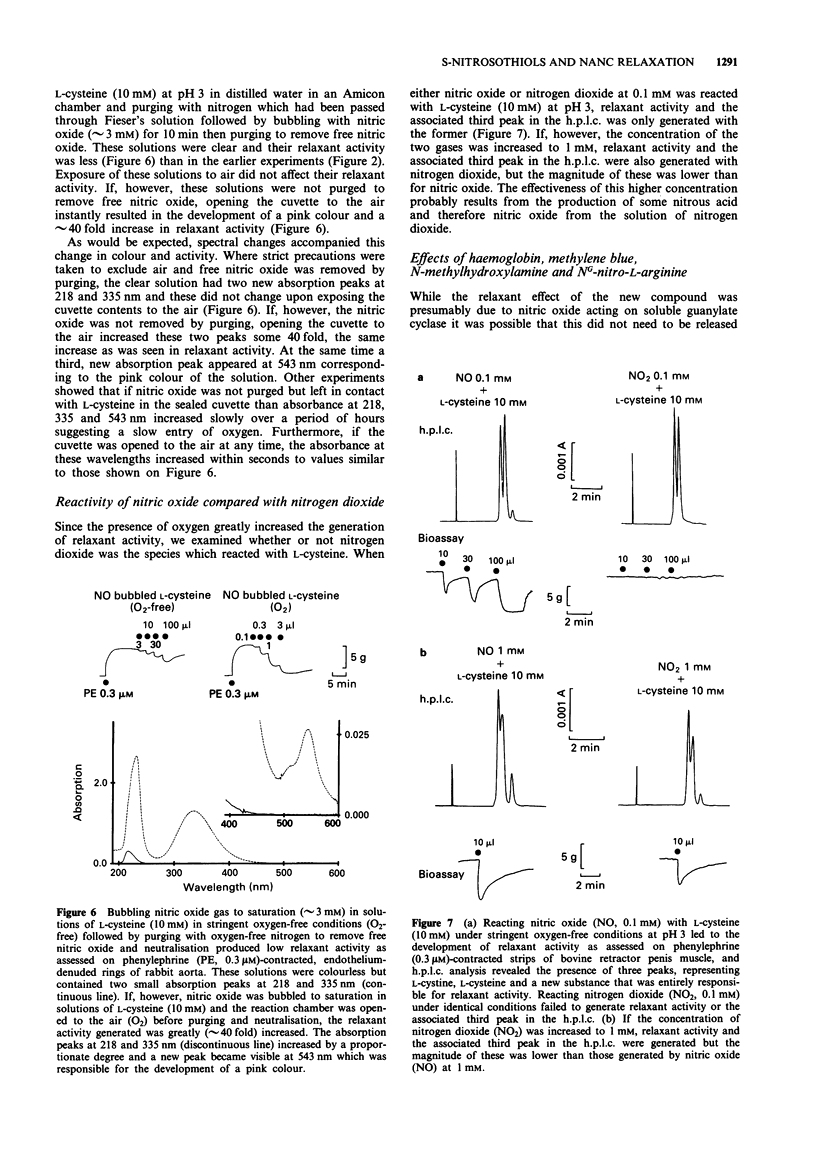
1. This study examined the possibility that an S-nitrosothiol, rather than nitric oxide, functions as the non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitter in the bovine retractor penis (BRP) muscle. 2. Treatment of BRP muscle with either of two sulphydryl inactivating agents, diamide (1 mM) and N-ethylmaleimide (0.3 mM), inhibited NANC relaxation and this was prevented by pretreating tissues with L-cysteine (3 mM), L-glutathione (3 mM) or dithiothreitol (3 mM). Inhibition was not specific, however, since the inactivating agents also inhibited the relaxant actions of authentic nitric oxide (0.3 microM), glyceryl trinitrate (0.001-1 microM) and isoprenaline (0.01-1 microM). 3. Reacting nitric oxide with L-cysteine in nominally oxygen-free solution at pH 3, followed by purging to remove free nitric oxide and neutralisation, produced greater and more prolonged relaxant activity when assayed on rabbit aortic rings than could be attributed to nitric oxide alone. H.p.l.c. analysis of the mixture identified a new peak distinct from either L-cysteine or nitric oxide which was responsible for the relaxant activity. The spectral absorption of this new compound had two bands with peaks at 218 and 335 nm. 4. Using a series of structural analogues of L-cysteine (all at 15 mM) it was found that removal of the carboxyl group (L-cysteamine), replacement of the carboxyl with an ester function (L-cysteine methyl ester) or substitution at the amino group (N-acetyl-L-cysteine) had no effect on the ability to generate relaxant activity upon reaction with nitric oxide (0.1 mM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeckxstaens G. E., Pelckmans P. A., Ruytjens I. F., Bult H., De Man J. G., Herman A. G., Van Maercke Y. M. Bioassay of nitric oxide released upon stimulation of non-adrenergic non-cholinergic nerves in the canine ileocolonic junction. Br J Pharmacol. 1991 May;103(1):1085–1091. doi: 10.1111/j.1476-5381.1991.tb12304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Soares-da-Silva P. A comparison of the action of the endothelium-derived relaxant factor and the inhibitory factor from the bovine retractor penis on rabbit aortic smooth muscle. Br J Pharmacol. 1986 Jan;87(1):175–181. doi: 10.1111/j.1476-5381.1986.tb10169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
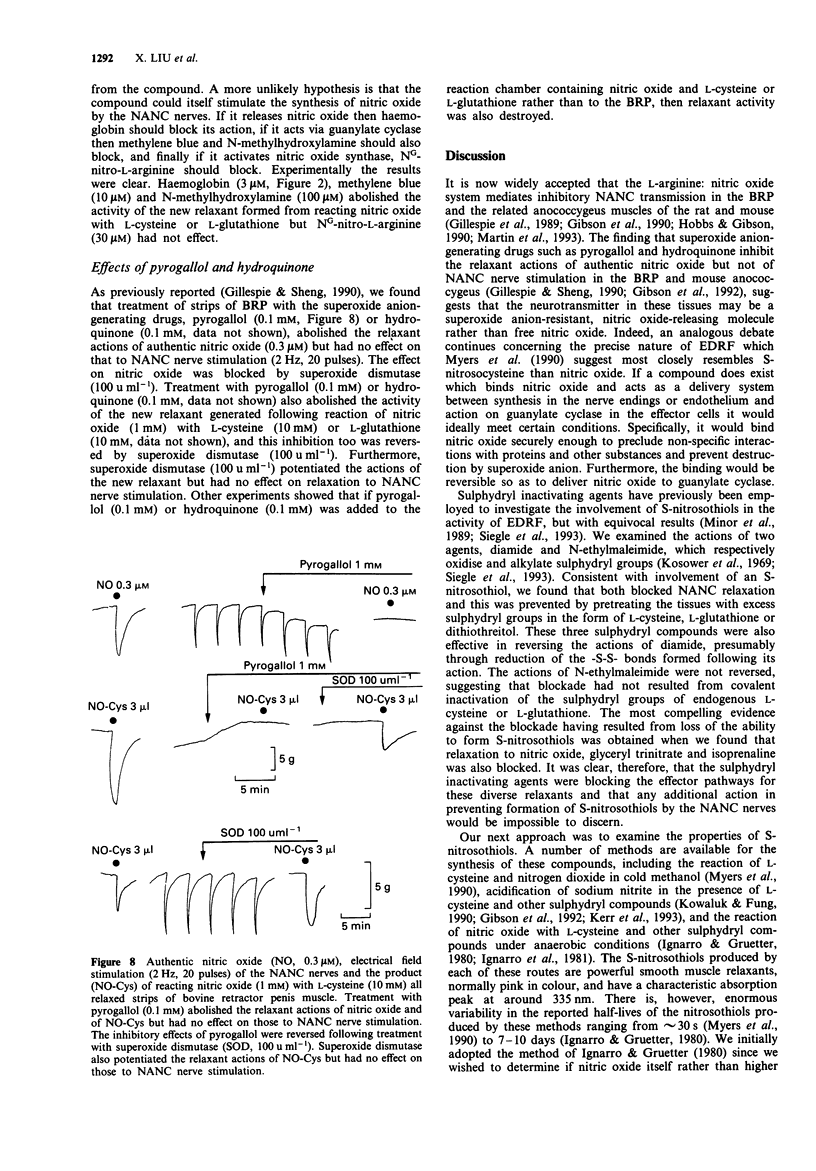
- Gillespie J. S., Sheng H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscles. Br J Pharmacol. 1990 Jan;99(1):194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. A., Schott C., Julou-Schaeffer G., Fleming I., Parratt J. R., Stoclet J. C. The effect of inhibitors of the L-arginine/nitric oxide pathway on endotoxin-induced loss of vascular responsiveness in anaesthetized rats. Br J Pharmacol. 1991 May;103(1):1218–1224. doi: 10.1111/j.1476-5381.1991.tb12327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Bush P. A., Buga G. M., Wood K. S., Fukuto J. M., Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990 Jul 31;170(2):843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Gruetter C. A. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim Biophys Acta. 1980 Aug 13;631(2):221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Kelm M., Schrader J. Nitric oxide release from the isolated guinea pig heart. Eur J Pharmacol. 1988 Oct 18;155(3):317–321. doi: 10.1016/0014-2999(88)90522-5. [DOI] [PubMed] [Google Scholar]
- Kerr S. W., Buchanan L. V., Bunting S., Mathews W. R. Evidence that S-nitrosothiols are responsible for the smooth muscle relaxing activity of the bovine retractor penis inhibitory factor. J Pharmacol Exp Ther. 1992 Oct;263(1):285–292. [PubMed] [Google Scholar]
- Kilbourn R. G., Griffith O. W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst. 1992 Jun 3;84(11):827–831. doi: 10.1093/jnci/84.11.827. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br J Pharmacol. 1991 Jan;102(1):91–94. doi: 10.1111/j.1476-5381.1991.tb12137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. R., Gillespie J. S., Gibson I. F., Martin W. Effects of NG-substituted analogues of L-arginine on NANC relaxation of the rat anococcygeus and bovine retractor penis muscles and the bovine penile artery. Br J Pharmacol. 1991 Sep;104(1):53–58. doi: 10.1111/j.1476-5381.1991.tb12384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Gillespie J. S., Gibson I. F. Actions and interactions of NG-substituted analogues of L-arginine on NANC neurotransmission in the bovine retractor penis and rat anococcygeus muscles. Br J Pharmacol. 1993 Jan;108(1):242–247. doi: 10.1111/j.1476-5381.1993.tb13469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Smith J. A., Lewis M. J., Henderson A. H. Evidence that inhibitory factor extracted from bovine retractor penis is nitrite, whose acid-activated derivative is stabilized nitric oxide. Br J Pharmacol. 1988 Mar;93(3):579–586. doi: 10.1111/j.1476-5381.1988.tb10313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Gryglewski R. J. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Moore P. K., Babbedge R. C., Wallace P., Gaffen Z. A., Hart S. L. 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br J Pharmacol. 1993 Feb;108(2):296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pickard R. S., Powell P. H., Zar M. A. The effect of inhibitors of nitric oxide biosynthesis and cyclic GMP formation on nerve-evoked relaxation of human cavernosal smooth muscle. Br J Pharmacol. 1991 Nov;104(3):755–759. doi: 10.1111/j.1476-5381.1991.tb12500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Modification by L-NG-monomethyl arginine (L-NMMA) of the response to nerve stimulation in isolated dog mesenteric and cerebral arteries. Jpn J Pharmacol. 1990 Jan;52(1):170–173. doi: 10.1254/jjp.52.170. [DOI] [PubMed] [Google Scholar]
- Tucker J. F., Brave S. R., Charalambous L., Hobbs A. J., Gibson A. L-NG-nitro arginine inhibits non-adrenergic, non-cholinergic relaxations of guinea-pig isolated tracheal smooth muscle. Br J Pharmacol. 1990 Aug;100(4):663–664. doi: 10.1111/j.1476-5381.1990.tb14072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Darbyshire J. F., Nims R. W., Saavedra J. E., Ford P. C. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993 Jan-Feb;6(1):23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- Yui Y., Ohkawa S., Ohnishi K., Hattori R., Aoyama T., Takahashi M., Morishita H., Terao Y., Kawai C. Mechanism for the generation of active smooth muscle inhibitory factor (IF) from bovine retractor penis muscle (BRP). Biochem Biophys Res Commun. 1989 Oct 16;164(1):544–549. doi: 10.1016/0006-291x(89)91753-1. [DOI] [PubMed] [Google Scholar]


