Abstract
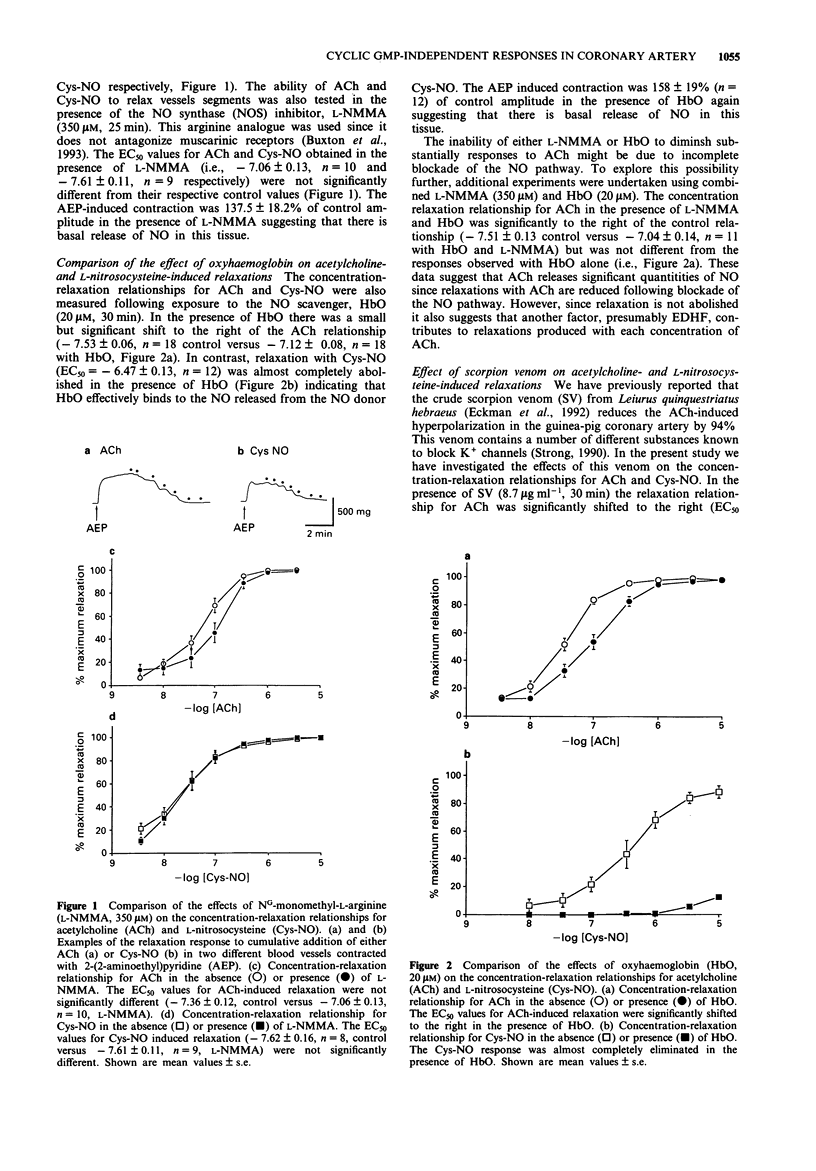
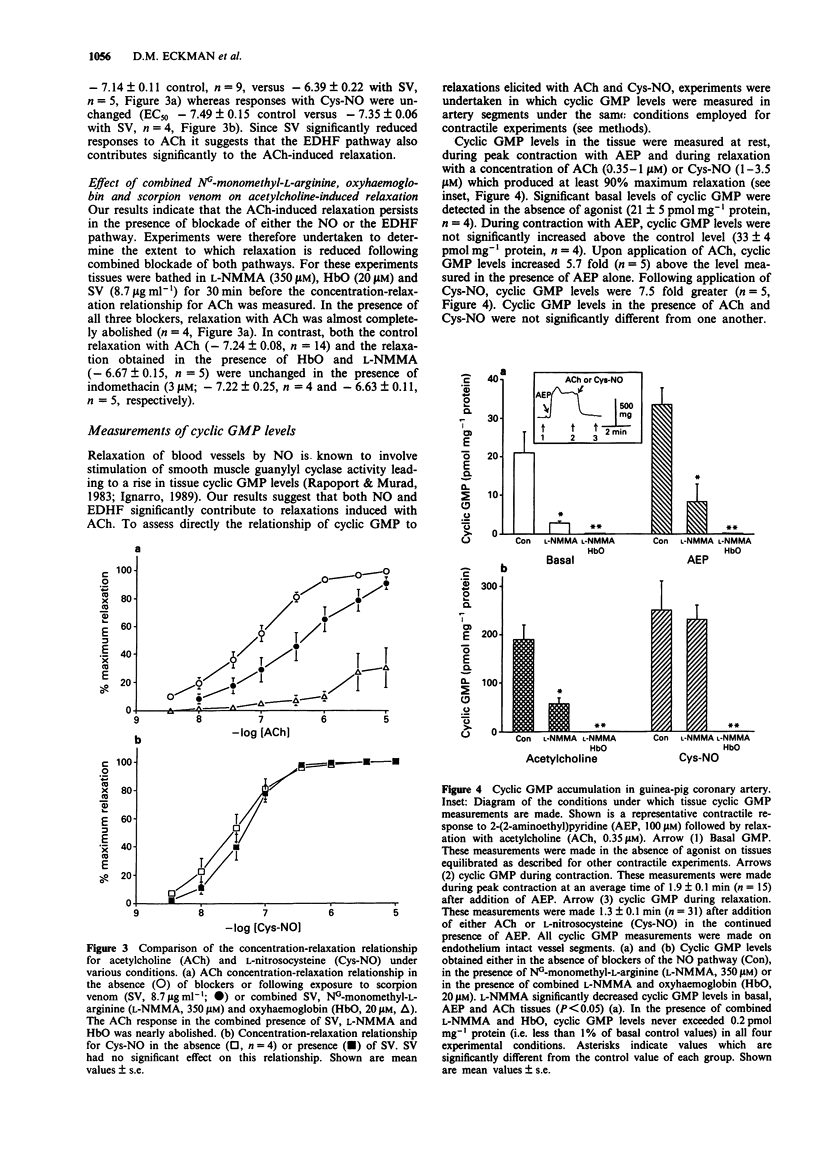
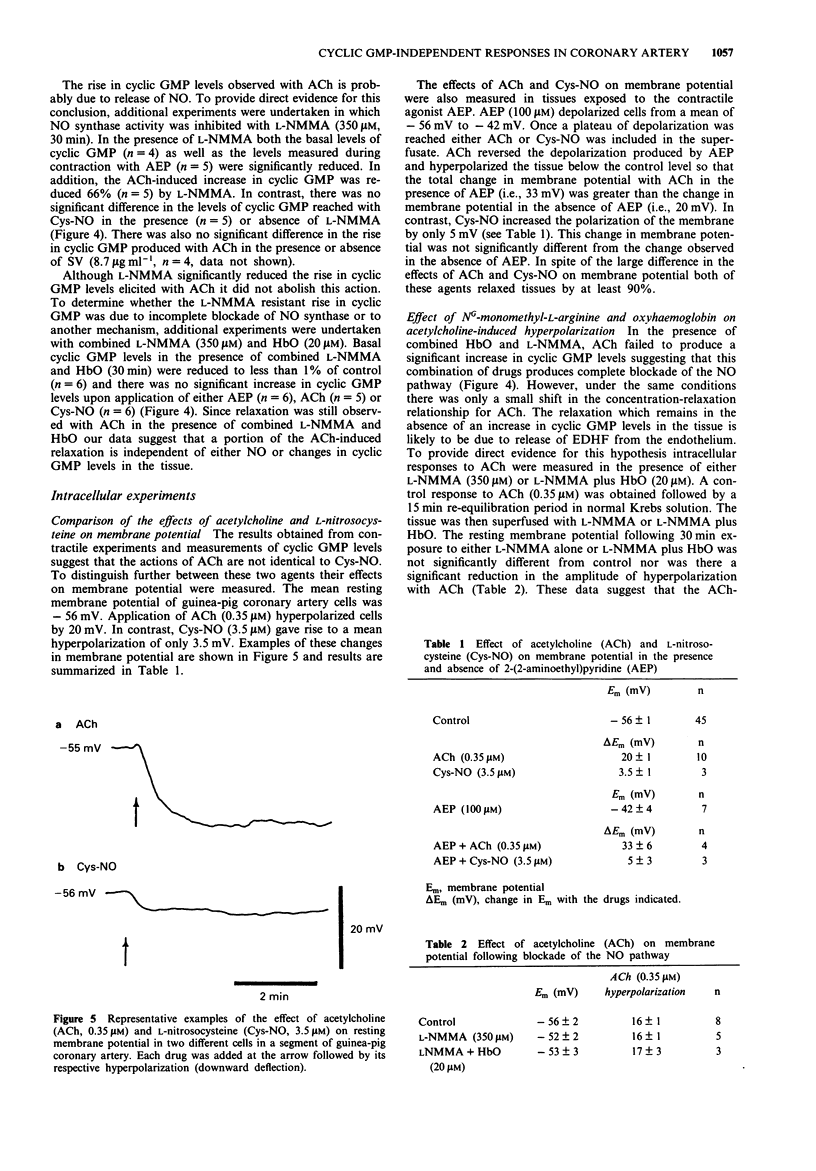
1. The effects of acetylcholine (ACh) on membrane potential, relaxation and cyclic GMP levels were compared to the NO donor L-nitrosocysteine (Cys-NO) in segments of guinea-pig coronary artery. 2. ACh and Cys-NO produced concentration-dependent relaxations of muscles contracted with the H1 receptor agonist, 2-(2-aminoethyl)pyridine (AEP, 0.35 mM). The relaxation to ACh was unchanged in the presence of NG-monomethyl-L-arginine (L-NMMA; 350 microM) or indomethacin (3 microM). 3. Oxyhaemoglobin (HbO; 20 microM) alone or in combination with L-NMMA increased the EC50 for ACh-induced relaxation whereas relaxation with Cys-NO was almost completely abolished with HbO. 4. Scorpion venom (SV; 8.7 micrograms ml-1) increased the EC50 for relaxation with ACh but not Cys-NO. Combined L-NMMA, HbO and SV produced nearly complete abolition of ACh-induced relaxations. 5. Basal cyclic GMP levels (i.e., 20 pmol mg-1 protein) were significantly increased following addition of either ACh (190 pmol mg-1 protein) or Cys-NO (240 pmol mg-1 protein). L-NMMA significantly reduced the rise of cyclic GMP with ACh but not Cys-NO. In contrast, SV did not significantly reduce the rise in cyclic GMP produced with ACh. In the combined presence of L-NMMA and HbO neither ACh nor Cys-NO produced a significant increase in cyclic GMP levels. 6. ACh gave rise to significantly greater membrane hyperpolarization than Cys-NO both in the presence and absence of AEP. Combined L-NMMA and HbO did not reduce the amplitude of hyperpolarization with ACh.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigaud M., Vatner S. F. Endothelium and blood flow mediated vasomotion in the conscious dog. Basic Res Cardiol. 1991;86 (Suppl 2):59–68. doi: 10.1007/978-3-642-72461-9_7. [DOI] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Block of some non-adrenergic inhibitory responses of smooth muscle by a substance from haemolysed erythrocytes. J Physiol. 1982 Jul;328:11–25. doi: 10.1113/jphysiol.1982.sp014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brayden J. E. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990 Sep;259(3 Pt 2):H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Quayle J. M., Standen N. B., Nelson M. T. Role of potassium channels in the vascular response to endogenous and pharmacological vasodilators. Blood Vessels. 1991;28(1-3):147–153. doi: 10.1159/000158854. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Cheek D. J., Eckman D., Westfall D. P., Sanders K. M., Keef K. D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993 Feb;72(2):387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C. Neither nitric oxide nor nitroglycerin accounts for all the characteristics of endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988;25(6):308–311. [PubMed] [Google Scholar]
- Chen G., Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol. 1989 Mar;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Yamamoto Y., Miwa K., Suzuki H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am J Physiol. 1991 Jun;260(6 Pt 2):H1888–H1892. doi: 10.1152/ajpheart.1991.260.6.H1888. [DOI] [PubMed] [Google Scholar]
- Dauphin F., Hamel E. Muscarinic receptor subtype mediating vasodilation feline middle cerebral artery exhibits M3 pharmacology. Eur J Pharmacol. 1990 Mar 20;178(2):203–213. doi: 10.1016/0014-2999(90)90476-m. [DOI] [PubMed] [Google Scholar]
- Durant G. J., Ganellin C. R., Parsons M. E. Chemical differentiation of histamine H1- and H2-receptor agonists. J Med Chem. 1975 Sep;18(9):905–909. doi: 10.1021/jm00243a009. [DOI] [PubMed] [Google Scholar]
- Eckman D. M., Frankovich J. D., Keef K. D. Comparison of the actions of acetylcholine and BRL 38227 in the guinea-pig coronary artery. Br J Pharmacol. 1992 May;106(1):9–16. doi: 10.1111/j.1476-5381.1992.tb14285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan N. A., Shimokawa H., Vanhoutte P. M. Pertussis toxin inhibits endothelium-dependent relaxations to certain agonists in porcine coronary arteries. J Physiol. 1989 Jan;408:549–560. doi: 10.1113/jphysiol.1989.sp017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Huang A. H., Busse R., Bassenge E. Endothelium-dependent hyperpolarization of smooth muscle cells in rabbit femoral arteries is not mediated by EDRF (nitric oxide). Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):438–442. doi: 10.1007/BF00172124. [DOI] [PubMed] [Google Scholar]
- Hynes M. R., Banner W., Jr, Yamamura H. I., Duckles S. P. Characterization of muscarinic receptors of the rabbit ear artery smooth muscle and endothelium. J Pharmacol Exp Ther. 1986 Jul;238(1):100–105. [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Wood K. S. Endothelium-dependent modulation of cGMP levels and intrinsic smooth muscle tone in isolated bovine intrapulmonary artery and vein. Circ Res. 1987 Jan;60(1):82–92. doi: 10.1161/01.res.60.1.82. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Keef K. D., Bowen S. M. Effect of ACh on electrical and mechanical activity in guinea pig coronary arteries. Am J Physiol. 1989 Oct;257(4 Pt 2):H1096–H1103. doi: 10.1152/ajpheart.1989.257.4.H1096. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kuriyama H. Effects of acetylcholine on the smooth muscle cell of isolated main coronary artery of the guinea-pig. J Physiol. 1979 Aug;293:119–133. doi: 10.1113/jphysiol.1979.sp012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Lian Q., Carl A., Kuriyama H. S-nitrosocysteine, but not sodium nitroprusside, produces apamin-sensitive hyperpolarization in rat gastric fundus. Br J Pharmacol. 1993 Jun;109(2):415–423. doi: 10.1111/j.1476-5381.1993.tb13585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Heterogeneous distribution of muscarinic receptors in the rabbit saphenous artery. Br J Pharmacol. 1987 Nov;92(3):657–664. doi: 10.1111/j.1476-5381.1987.tb11369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K., Vanhoutte P. M. Endothelium-derived hyperpolarizing factor. Blood Vessels. 1990;27(2-5):238–245. doi: 10.1159/000158815. [DOI] [PubMed] [Google Scholar]
- Malinowska B., Schlicker E. Identification of endothelial H1, vascular H2 and cardiac presynaptic H3 receptors in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jan;347(1):55–60. doi: 10.1007/BF00168772. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- McPherson G. A., Angus J. A. Evidence that acetylcholine-mediated hyperpolarization of the rat small mesenteric artery does not involve the K+ channel opened by cromakalim. Br J Pharmacol. 1991 May;103(1):1184–1190. doi: 10.1111/j.1476-5381.1991.tb12321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisheri K. D., Cipkus L. A., Taylor C. J. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988 Jun;245(3):751–760. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization contributes to endothelium-dependent relaxations to acetylcholine in femoral veins of rats. Am J Physiol. 1991 Oct;261(4 Pt 2):H1034–H1037. doi: 10.1152/ajpheart.1991.261.4.H1034. [DOI] [PubMed] [Google Scholar]
- Nishiye E., Nakao K., Itoh T., Kuriyama H. Factors inducing endothelium-dependent relaxation in the guinea-pig basilar artery as estimated from the actions of haemoglobin. Br J Pharmacol. 1989 Mar;96(3):645–655. doi: 10.1111/j.1476-5381.1989.tb11864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Strong P. N. Potassium channel toxins. Pharmacol Ther. 1990;46(1):137–162. doi: 10.1016/0163-7258(90)90040-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Chen G., Yamamoto Y., Miwa K. Nitroarginine-sensitive and -insensitive components of the endothelium-dependent relaxation in the guinea-pig carotid artery. Jpn J Physiol. 1992;42(2):335–347. doi: 10.2170/jjphysiol.42.335. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Weir S. W., Weston A. H. The effects of BRL 34915 and nicorandil on electrical and mechanical activity and on 86Rb efflux in rat blood vessels. Br J Pharmacol. 1986 May;88(1):121–128. doi: 10.1111/j.1476-5381.1986.tb09478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


