Abstract
1. The effects of myocardial ischaemia/reperfusion were tested on the coronary vasorelaxant responses to agonists selective for the A1 and A2 adenosine receptor subtypes in the dog. The left anterior descending (LAD) coronary artery was occluded distal to the first diagonal branch. The occlusion was maintained for 1 h, followed by 1 h of reperfusion. 2. In the first series of experiments, LAD and circumflex arteries were excised and contracted with prostaglandin F2 alpha (PGF2 alpha). Ischaemia/reperfusion did not significantly alter the vasorelaxation produced by either sodium nitroprusside (endothelium-independent) or acetylcholine (endothelium-dependent). The A1 selective agonist, cyclopentyladenosine (CPA), produced coronary vasorelaxation in both normally perfused vessels and vessels subjected to ischaemia/reperfusion. In contrast, the relaxation produced by the A2-selective agonist N6-[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl) ethyl] adenosine (DPMA) was significantly attenuated by ischaemia/reperfusion (14 fold shift in EC50). 3. In the second series of experiments, coronary blood flow was increased by administration of the A1 and A2 agonists before and after ischaemia/reperfusion of the LAD in anaesthetized dogs. Both compounds dose-dependently increased coronary blood flow. The slopes of the dose-response functions to CPA or DPMA were not significantly altered in the normally perfused circumflex vascular bed. Similarly, the CPA dose-response function in the LAD was unaltered by ischaemia/reperfusion. However, the slope of the coronary vasodilator response to the A2 agonist was significantly reduced following ischaemia/reperfusion of the LAD. 4. We conclude that ischaemia/reperfusion reduces responsiveness to an adenosine A2 receptor subtype agonist, but not an A1 receptor subtype agonist. These data confirm the independent nature of A1- and A2-mediated coronary vasodilatation.
Full text
PDF

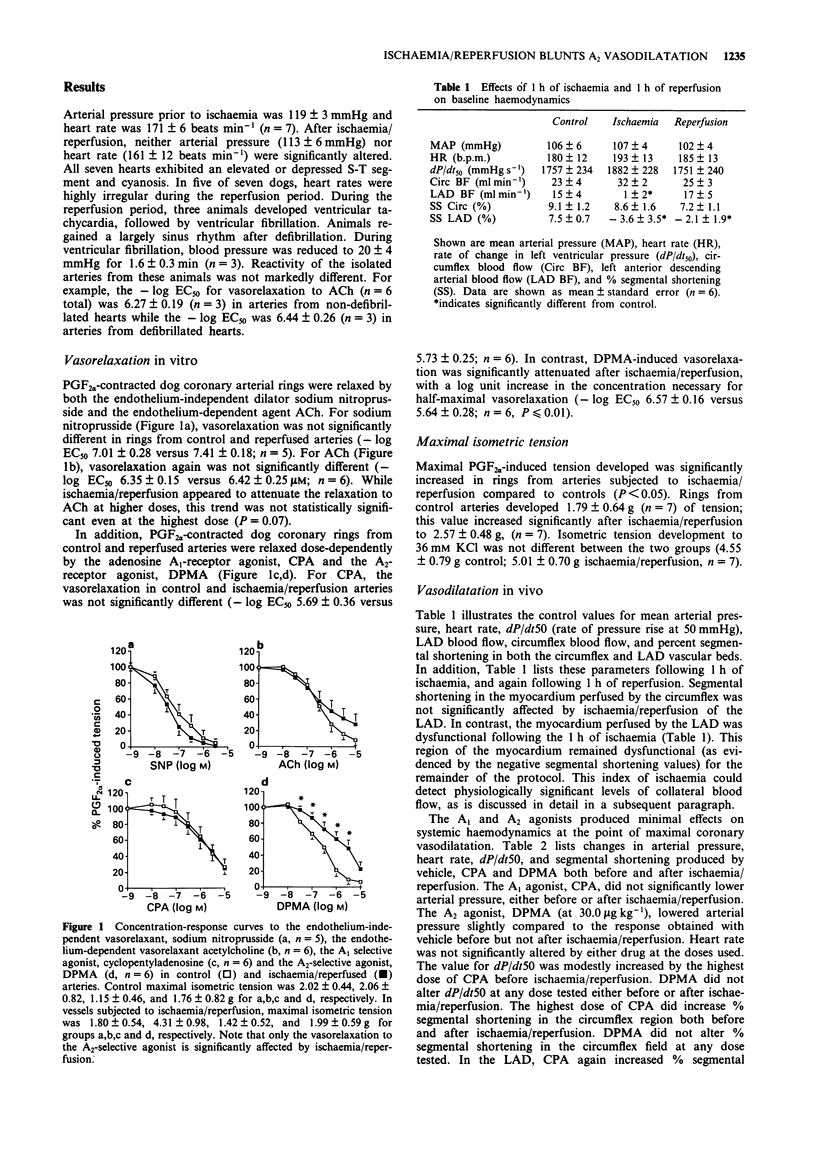
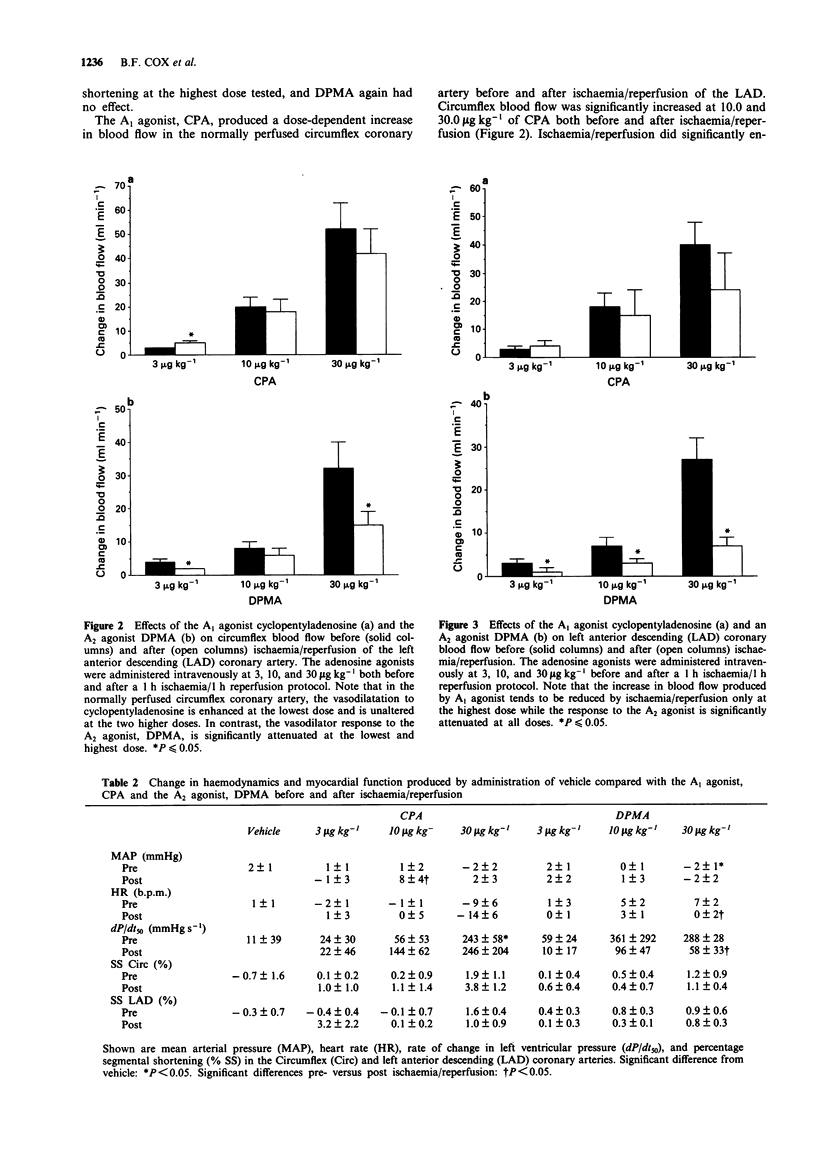



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchampach J. A., Maruyama M., Cavero I., Gross G. J. The new K+ channel opener Aprikalim (RP 52891) reduces experimental infarct size in dogs in the absence of hemodynamic changes. J Pharmacol Exp Ther. 1991 Dec;259(3):961–967. [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Bolli R., Triana J. F., Jeroudi M. O. Prolonged impairment of coronary vasodilation after reversible ischemia. Evidence for microvascular "stunning". Circ Res. 1990 Aug;67(2):332–343. doi: 10.1161/01.res.67.2.332. [DOI] [PubMed] [Google Scholar]
- Bolli R., Zhu W. X., Thornby J. I., O'Neill P. G., Roberts R. Time course and determinants of recovery of function after reversible ischemia in conscious dogs. Am J Physiol. 1988 Jan;254(1 Pt 2):H102–H114. doi: 10.1152/ajpheart.1988.254.1.H102. [DOI] [PubMed] [Google Scholar]
- Cowley M. J., Dorros G., Kelsey S. F., Van Raden M., Detre K. M. Acute coronary events associated with percutaneous transluminal coronary angioplasty. Am J Cardiol. 1984 Jun 15;53(12):12C–16C. doi: 10.1016/0002-9149(84)90738-0. [DOI] [PubMed] [Google Scholar]
- Deussen A., Borst M., Kroll K., Schrader J. Formation of S-adenosylhomocysteine in the heart. II: A sensitive index for regional myocardial underperfusion. Circ Res. 1988 Jul;63(1):250–261. doi: 10.1161/01.res.63.1.250. [DOI] [PubMed] [Google Scholar]
- Gerencer R. Z., Finegan B. A., Clanachan A. S. Cardiovascular selectivity of adenosine receptor agonists in anaesthetized dogs. Br J Pharmacol. 1992 Dec;107(4):1048–1056. doi: 10.1111/j.1476-5381.1992.tb13405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover G. J., Dzwonczyk S., Sleph P. G. Reduction of ischemic damage in isolated rat hearts by the potassium channel opener, RP 52891. Eur J Pharmacol. 1990 Nov 20;191(1):11–18. doi: 10.1016/0014-2999(90)94091-b. [DOI] [PubMed] [Google Scholar]
- Heyndrickx G. R., Millard R. W., McRitchie R. J., Maroko P. R., Vatner S. F. Regional myocardial functional and electrophysiological alterations after brief coronary artery occlusion in conscious dogs. J Clin Invest. 1975 Oct;56(4):978–985. doi: 10.1172/JCI108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M. F., Williams M., Do U. H., Sills M. A. Characterization of the binding of a novel nonxanthine adenosine antagonist radioligand, [3H]CGS 15943, to multiple affinity states of the adenosine A1 receptor in the rat cortex. Mol Pharmacol. 1991 Jan;39(1):49–54. [PubMed] [Google Scholar]
- Jennings R. B., Schaper J., Hill M. L., Steenbergen C., Jr, Reimer K. A. Effect of reperfusion late in the phase of reversible ischemic injury. Changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ Res. 1985 Feb;56(2):262–278. doi: 10.1161/01.res.56.2.262. [DOI] [PubMed] [Google Scholar]
- Ku D. D. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982 Nov 5;218(4572):576–578. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- Lasley R. D., Rhee J. W., Van Wylen D. G., Mentzer R. M., Jr Adenosine A1 receptor mediated protection of the globally ischemic isolated rat heart. J Mol Cell Cardiol. 1990 Jan;22(1):39–47. doi: 10.1016/0022-2828(90)90970-d. [DOI] [PubMed] [Google Scholar]
- Merkel L. A., Lappe R. W., Rivera L. M., Cox B. F., Perrone M. H. Demonstration of vasorelaxant activity with an A1-selective adenosine agonist in porcine coronary artery: involvement of potassium channels. J Pharmacol Exp Ther. 1992 Feb;260(2):437–443. [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Pearson P. J., Schaff H. V., Vanhoutte P. M. Acute impairment of endothelium-dependent relaxations to aggregating platelets following reperfusion injury in canine coronary arteries. Circ Res. 1990 Aug;67(2):385–393. doi: 10.1161/01.res.67.2.385. [DOI] [PubMed] [Google Scholar]
- Quillen J. E., Sellke F. W., Brooks L. A., Harrison D. G. Ischemia-reperfusion impairs endothelium-dependent relaxation of coronary microvessels but does not affect large arteries. Circulation. 1990 Aug;82(2):586–594. doi: 10.1161/01.cir.82.2.586. [DOI] [PubMed] [Google Scholar]
- Ramkumar V., Olah M. E., Jacobson K. A., Stiles G. L. Distinct pathways of desensitization of A1- and A2-adenosine receptors in DDT1 MF-2 cells. Mol Pharmacol. 1991 Nov;40(5):639–647. [PMC free article] [PubMed] [Google Scholar]
- Suba E. A., Roth B. L. Prostaglandins activate phosphoinositide metabolism in rat aorta. Eur J Pharmacol. 1987 Apr 29;136(3):325–332. doi: 10.1016/0014-2999(87)90305-0. [DOI] [PubMed] [Google Scholar]
- Thornton J. D., Liu G. S., Olsson R. A., Downey J. M. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation. 1992 Feb;85(2):659–665. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- Toombs C. F., McGee S., Johnston W. E., Vinten-Johansen J. Myocardial protective effects of adenosine. Infarct size reduction with pretreatment and continued receptor stimulation during ischemia. Circulation. 1992 Sep;86(3):986–994. doi: 10.1161/01.cir.86.3.986. [DOI] [PubMed] [Google Scholar]
- VanBenthuysen K. M., McMurtry I. F., Horwitz L. D. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J Clin Invest. 1987 Jan;79(1):265–274. doi: 10.1172/JCI112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. Y., Li C., Olah M. E., Johnson R. A., Stiles G. L., Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R., Ronca-Testoni S., Galbani P., Yu G., Mariani M., Ronca G. Cardiac A2 adenosine receptors--influence of ischaemia. Cardiovasc Res. 1992 May;26(5):549–554. doi: 10.1093/cvr/26.5.549. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


