Abstract
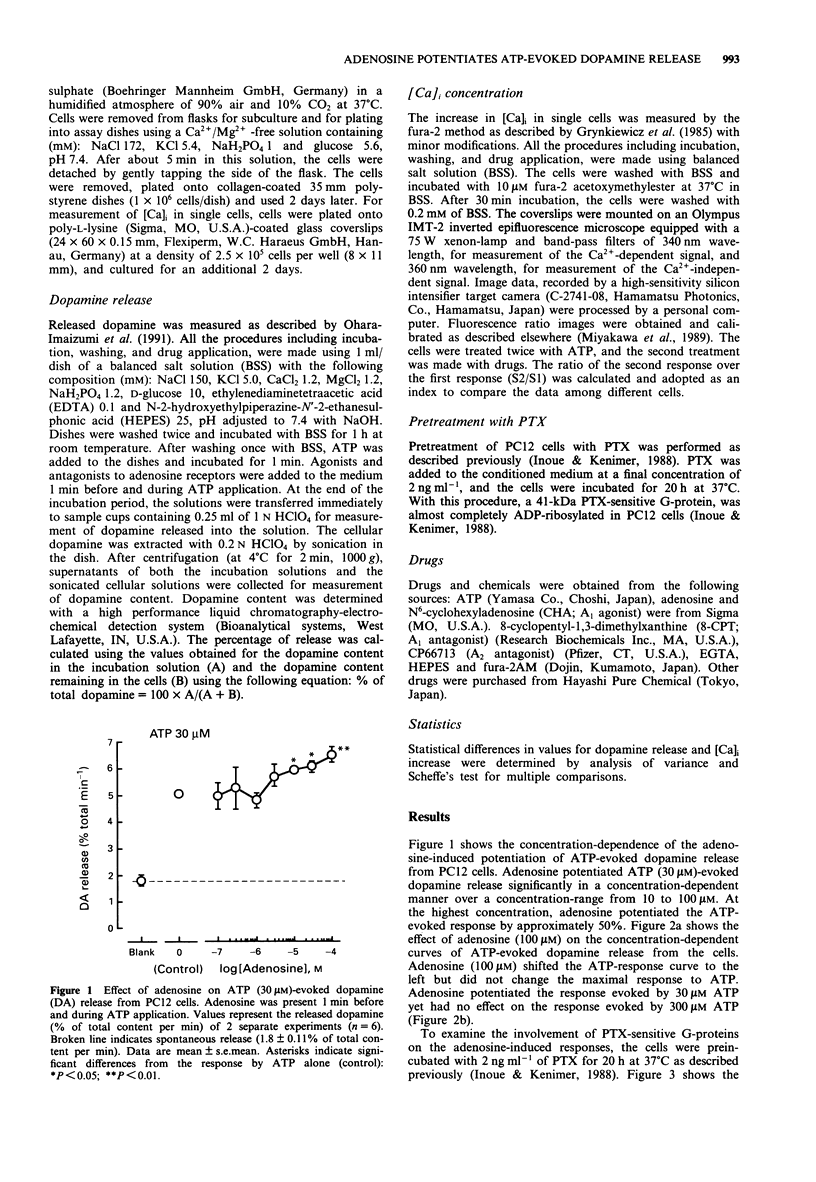
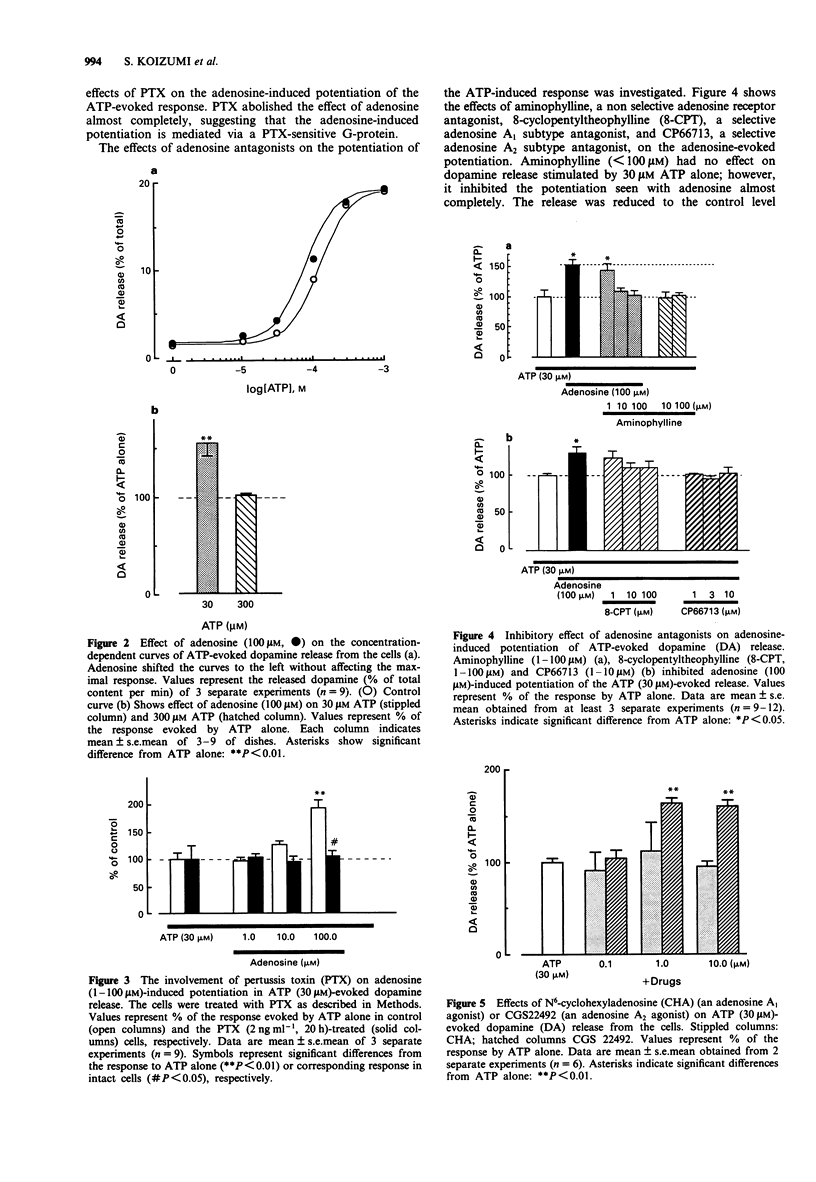
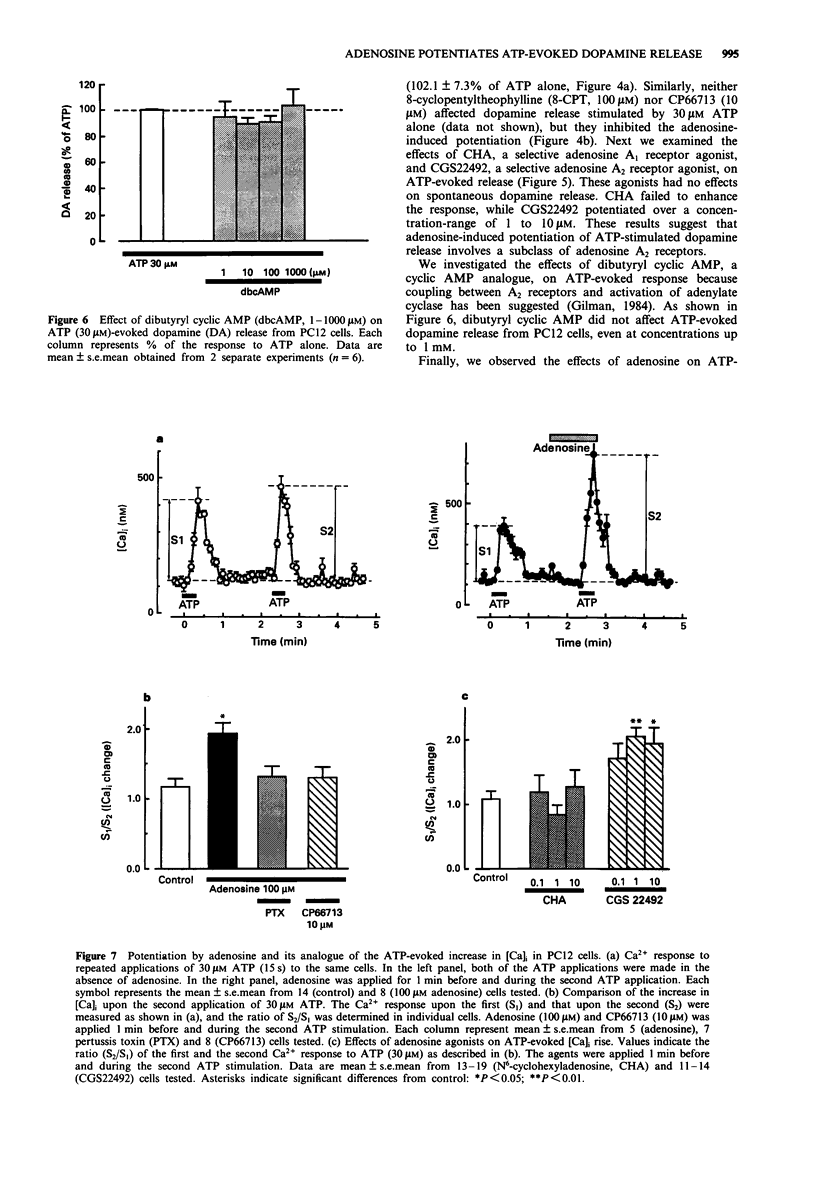
1. The effects of adenosine on adenosine 5'-triphosphate (ATP)-evoked dopamine release from rat phaeochromocytoma PC12 cells was investigated to determine whether adenosine exerts a regulatory effect on the ATP-evoked response. Adenosine potentiated ATP (30 microM)-evoked dopamine release in a concentration-dependent manner over a concentration-range of 1 to 100 microM. Adenosine (100 microM) shifted the concentration-dependence of the ATP-evoked response to the left without affecting the maximal response. 2. Aminophylline, a non-selective adenosine receptor antagonist, and CP66713, a selective antagonist at the A2 subclass of adenosine receptors, abolished the adenosine-induced potentiation. Furthermore, 8-cyclopentyltheophylline, a selective antagonist at the adenosine A1 receptor partially inhibited the adenosine-evoked potentiation. CGS22492, a selective A2 receptor agonist, potentiated ATP-evoked dopamine release whereas N6-cyclohexyladenosine (CHA), a selective A1 receptor agonist, had no effect. 3. Pertussis toxin (PTX), a bacterial exotoxin which catalyzes the ADP-ribosylation of guanosine 5'-triphosphate (GTP)-binding proteins (G-proteins), inhibited the adenosine-induced potentiation of dopamine release. Dibutyryl cyclic AMP (db cyclic AMP), an analogue of cyclic AMP, had no effect on the release on the ATP-evoked response. 4. Adenosine potentiated the ATP-evoked rise in intracellular Ca2+ concentration ([Ca]i) in PC12 cells. This potentiation was also observed with CGS 22492 but not with CHA. PTX completely inhibited the adenosine-induced potentiation of the rise in [Ca]i. 5. On the basis of these findings, we suggest that the adenosine-induced potentiation of ATP-evoked dopamine release was due to an increase in [Ca]i in the cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. A dual function for adenosine 5'-triphosphate in the regulation of vascular tone. Excitatory cotransmitter with noradrenaline from perivascular nerves and locally released inhibitory intravascular agent. Circ Res. 1986 Mar;58(3):319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J. ATP--a fast neurotransmitter. FEBS Lett. 1993 Jun 28;325(1-2):86–89. doi: 10.1016/0014-5793(93)81419-z. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Gerwins P., Fredholm B. B. ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J Biol Chem. 1992 Aug 15;267(23):16081–16087. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hide I., Padgett W. L., Jacobson K. A., Daly J. W. A2A adenosine receptors from rat striatum and rat pheochromocytoma PC12 cells: characterization with radioligand binding and by activation of adenylate cyclase. Mol Pharmacol. 1992 Feb;41(2):352–359. [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Kenimer J. G. Muscarinic stimulation of calcium influx and norepinephrine release in PC12 cells. J Biol Chem. 1988 Jun 15;263(17):8157–8161. [PubMed] [Google Scholar]
- Inoue K., Nakazawa K., Fujimori K., Takanaka A. Extracellular adenosine 5'-triphosphate-evoked norepinephrine secretion not relating to voltage-gated Ca channels in pheochromocytoma PC12 cells. Neurosci Lett. 1989 Dec 4;106(3):294–299. doi: 10.1016/0304-3940(89)90179-1. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Gatti G., Ambrosini A., Pozzan T., Westhead E. W. Second-messenger control of catecholamine release from PC12 cells. Role of muscarinic receptors and nerve-growth-factor-induced cell differentiation. Biochem J. 1988 Nov 1;255(3):761–768. doi: 10.1042/bj2550761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Inoue K. Roles of Ca2+ influx through ATP-activated channels in catecholamine release from pheochromocytoma PC12 cells. J Neurophysiol. 1992 Dec;68(6):2026–2032. doi: 10.1152/jn.1992.68.6.2026. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Watano T., Inoue K. Mechanisms underlying facilitation by dopamine of ATP-activated currents in rat pheochromocytoma cells. Pflugers Arch. 1993 Feb;422(5):458–464. doi: 10.1007/BF00375072. [DOI] [PubMed] [Google Scholar]
- Nazarea M., Okajima F., Kondo Y. P2-purinergic activation of phosphoinositide turnover is potentiated by A1-receptor stimulation in thyroid cells. Eur J Pharmacol. 1991 Jan 25;206(1):47–52. doi: 10.1016/0922-4106(91)90145-8. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M., Nakazawa K., Obama T., Fujimori K., Takanaka A., Inoue K. Inhibitory action of peripheral-type benzodiazepines on dopamine release from PC12 pheochromocytoma cells. J Pharmacol Exp Ther. 1991 Nov;259(2):484–489. [PubMed] [Google Scholar]
- Okada Y., Kuroda Y. Inhibitory action of adenosine and adenosine analogs on neurotransmission in the olfactory cortex slice of guinea pig - structure-activity relationships. Eur J Pharmacol. 1980 Jan 25;61(2):137–146. doi: 10.1016/0014-2999(80)90156-9. [DOI] [PubMed] [Google Scholar]
- Okajima F., Sato K., Nazarea M., Sho K., Kondo Y. A permissive role of pertussis toxin substrate G-protein in P2-purinergic stimulation of phosphoinositide turnover and arachidonate release in FRTL-5 thyroid cells. Cooperative mechanism of signal transduction systems. J Biol Chem. 1989 Aug 5;264(22):13029–13037. [PubMed] [Google Scholar]
- Phillis J. W., Kostopoulos G. K., Limacher J. J. A potent depressant action of adenine derivatives on cerebral cortical neurones. Eur J Pharmacol. 1975 Jan;30(1):125–129. doi: 10.1016/0014-2999(75)90214-9. [DOI] [PubMed] [Google Scholar]
- Stiles G. L. Adenosine receptors. J Biol Chem. 1992 Apr 5;267(10):6451–6454. [PubMed] [Google Scholar]


