Abstract
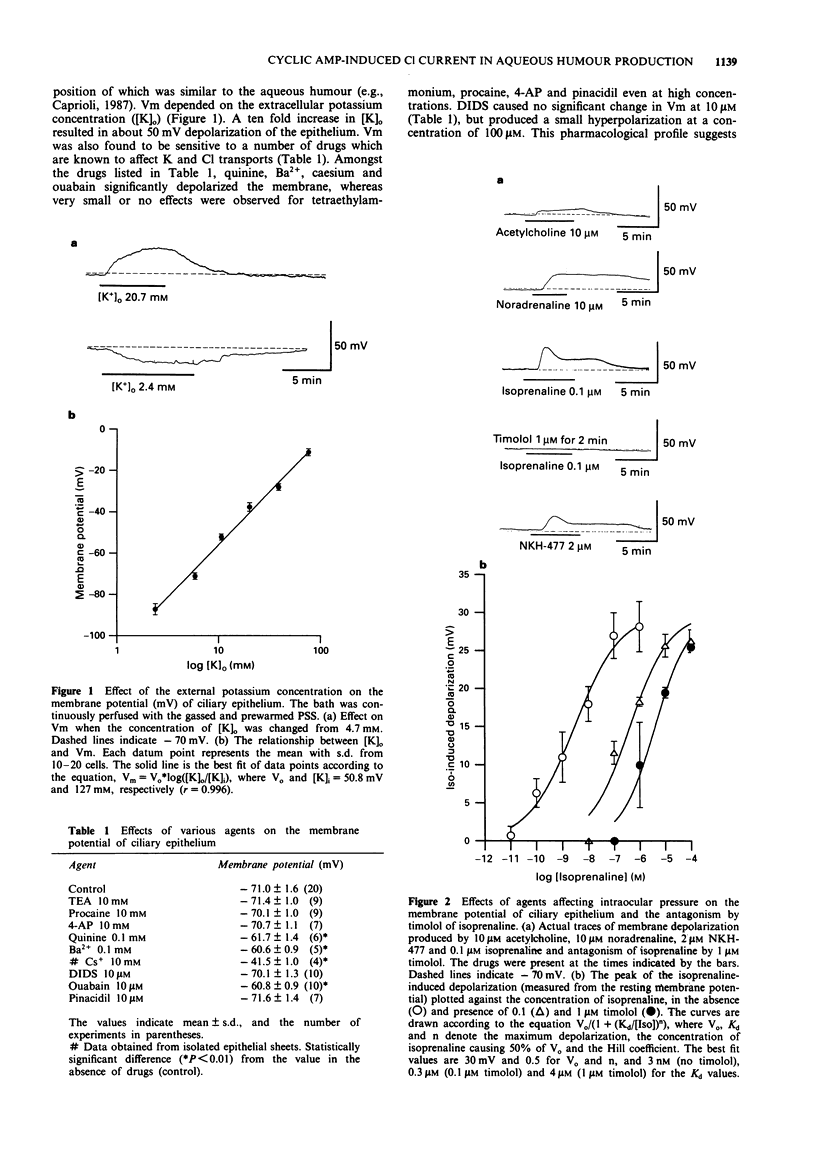
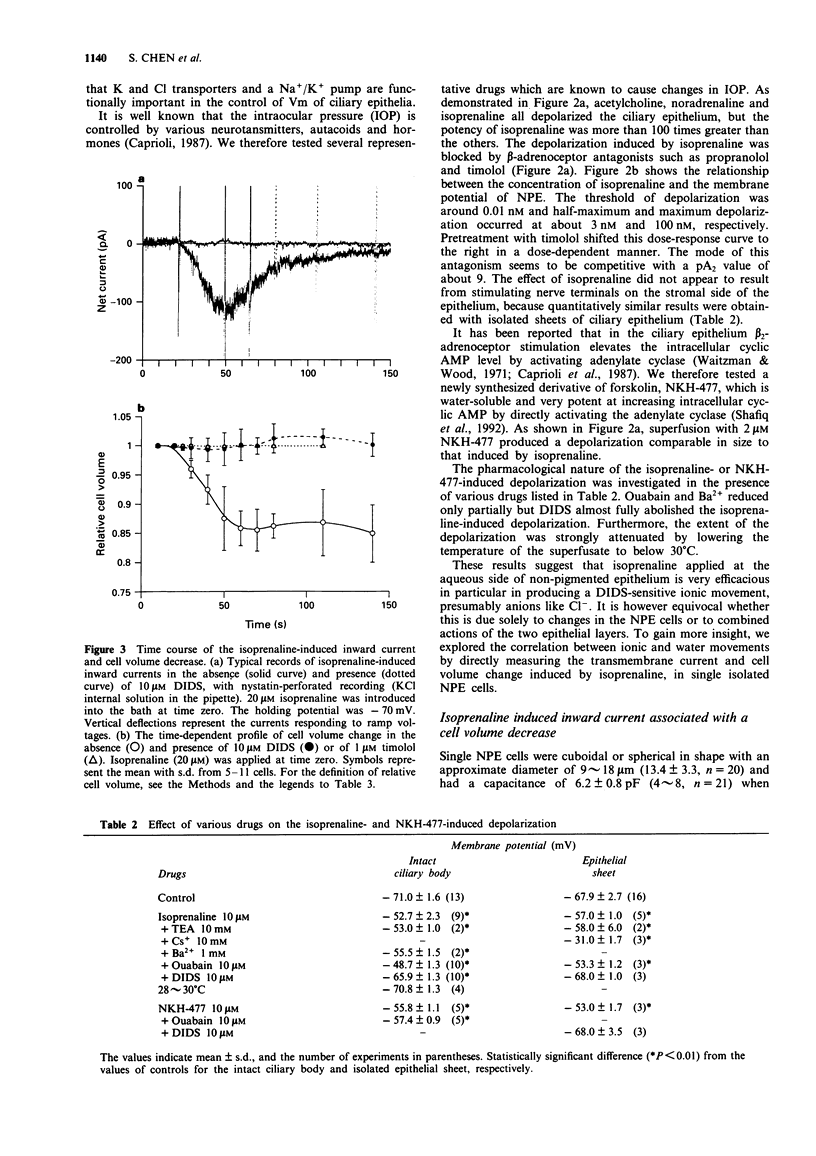
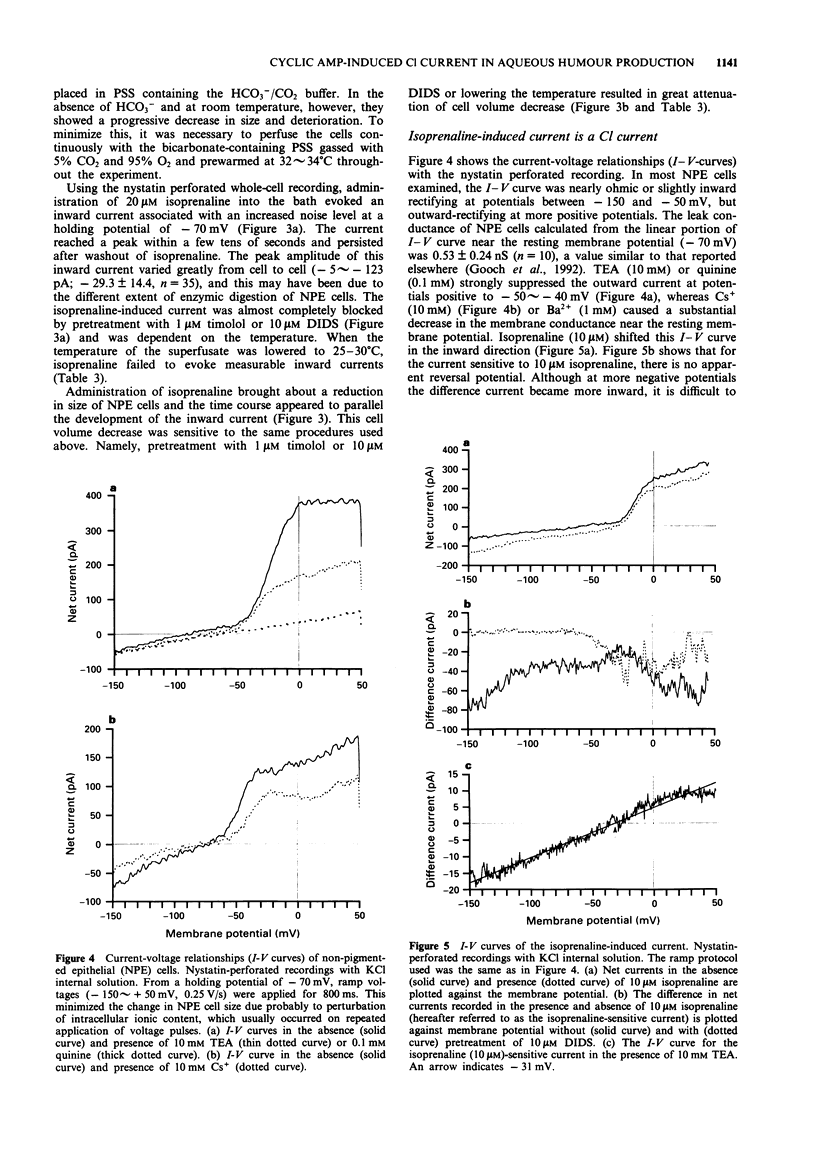
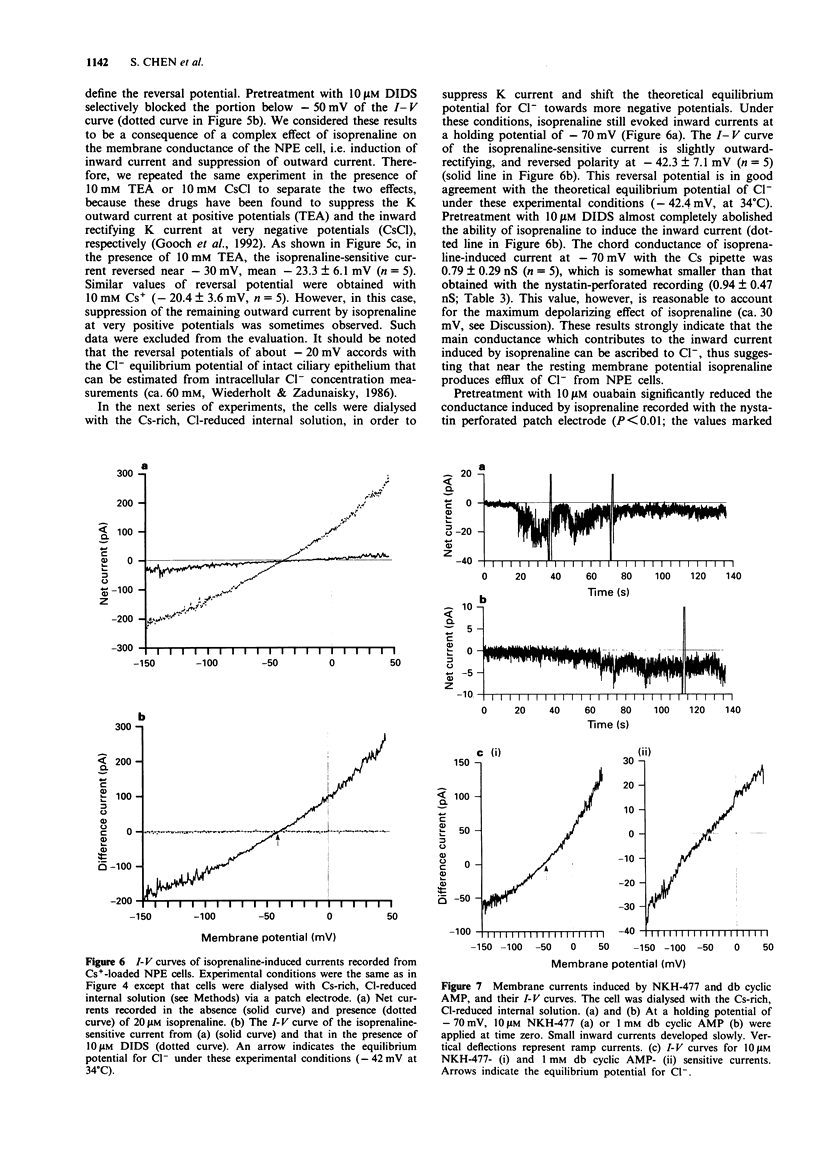
1. The effects of isoprenaline, a forskolin derivative NKH-477, and dibutyryl cyclic AMP (db cyclic AMP) on the membrane potential, conductance and cell volume of the dog non-pigmented ciliary epithelium (NPE) were investigated by intracellular potential recording, nystatin-perforated patch clamp technique and videomicroscopic cytometry. 2. The resting membrane potential of NPE was about -70 mV in physiological saline and was depolarized by isoprenaline in a dose-dependent manner with an ED50 of about 3 nM. This depolarization was competitively antagonized by the beta-adrenoceptor antagonist, timolol (pA2 = ca. 9) and almost completely blocked by the Cl transport blocker, DIDS. 3. In single dissociated NPE cells, 10 microM isoprenaline induced an inward current and caused a concomitant decrease in cell volume. The reversal potential measurement indicated that this inward current was carried mainly by Cl ion. DIDS (10 microM) abolished both the current and cell volume decrease. 4. NKH-477 (10 microM) or db cyclic AMP (1 mM) also induced an inward current together with a cell volume decrease, the properties of which were similar to those caused by isoprenaline. 5. These results suggest that beta-adrenoceptor stimulation in NPE leads to an increased rate of aqueous humour production by increasing Cl- efflux via an elevation of cyclic AMP and this effect is efficiently blocked by timolol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartels S. P., Lee S. R., Neufeld A. H. Forskolin stimulates cyclic AMP synthesis, lowers intraocular pressure and increases outflow facility in rabbits. Curr Eye Res. 1982;2(10):673–681. doi: 10.3109/02713688209019996. [DOI] [PubMed] [Google Scholar]
- Caprioli J., Sears M., Mead A., Kosley R. W., Jr, Cherill R. J., Hugher F. P., Allen R. C., Tressler C. Adenylate cyclase stimulation and intraocular pressure reduction by forskolin analogs. J Ocul Pharmacol. 1989 Fall;5(3):181–187. doi: 10.1089/jop.1989.5.181. [DOI] [PubMed] [Google Scholar]
- Chen S., Inoue R., Ito Y. Pharmacological characterization of muscarinic receptor-activated cation channels in guinea-pig ileum. Br J Pharmacol. 1993 Jul;109(3):793–801. doi: 10.1111/j.1476-5381.1993.tb13644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D. F. ELECTRICAL POTENTIAL ACROSS THE ISOLATED CILIARY BODY OBSERVED IN VITRO. Br J Ophthalmol. 1961 Oct;45(10):641–653. doi: 10.1136/bjo.45.10.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D. C. Ion channels and colonic salt transport. Annu Rev Physiol. 1991;53:321–339. doi: 10.1146/annurev.ph.53.030191.001541. [DOI] [PubMed] [Google Scholar]
- De Weer P., Gadsby D. C., Rakowski R. F. Voltage dependence of the Na-K pump. Annu Rev Physiol. 1988;50:225–241. doi: 10.1146/annurev.ph.50.030188.001301. [DOI] [PubMed] [Google Scholar]
- Delamere N. A., Socci R. R., King K. L. Alteration of sodium, potassium-adenosine triphosphatase activity in rabbit ciliary processes by cyclic adenosine monophosphate-dependent protein kinase. Invest Ophthalmol Vis Sci. 1990 Oct;31(10):2164–2170. [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahbakhsh N. A., Fain G. L. Volume regulation of non-pigmented cells from ciliary epithelium. Invest Ophthalmol Vis Sci. 1987 Jun;28(6):934–944. [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Gooch A. J., Morgan J., Jacob T. J. Adrenergic stimulation of bovine non-pigmented ciliary epithelial cells raises cAMP but has no effect on K+ or Cl- currents. Curr Eye Res. 1992 Oct;11(10):1019–1029. doi: 10.3109/02713689209033500. [DOI] [PubMed] [Google Scholar]
- Holland M. G., Gipson C. C. Chloride ion transport in the isolated ciliary body. Invest Ophthalmol. 1970 Jan;9(1):20–29. [PubMed] [Google Scholar]
- Hughes B. A., Segawa Y. cAMP-activated chloride currents in amphibian retinal pigment epithelial cells. J Physiol. 1993 Jul;466:749–766. [PMC free article] [PubMed] [Google Scholar]
- Iizuka S., Kishida K., Tsuboi S., Emi K., Manabe R. Electrical characteristics of the isolated dog ciliary body. Curr Eye Res. 1984 Mar;3(3):417–421. doi: 10.3109/02713688408997228. [DOI] [PubMed] [Google Scholar]
- Katz I. M., Hubbard W. A., Getson A. J., Gould A. L. Intraocular pressure decrease in normal volunteers following timolol ophthalmic solution. Invest Ophthalmol. 1976 Jun;15(6):489–492. [PubMed] [Google Scholar]
- Kishida K., Sasabe T., Iizuka S., Manabe R., Otori T. Sodium and chloride transport across the isolated rabbit ciliary body. Curr Eye Res. 1982;2(3):149–152. doi: 10.3109/02713688208997688. [DOI] [PubMed] [Google Scholar]
- Klyce S. D., Wong R. K. Site and mode of adrenaline action on chloride transport across the rabbit corneal epithelium. J Physiol. 1977 Apr;266(3):777–799. doi: 10.1113/jphysiol.1977.sp011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Vasallo P., Ghosh S., Coca-Prados M. Expression of Na,K-ATPase alpha subunit isoforms in the human ciliary body and cultured ciliary epithelial cells. J Cell Physiol. 1989 Nov;141(2):243–252. doi: 10.1002/jcp.1041410203. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Regulation of Cl- and K+ channels in airway epithelium. Annu Rev Physiol. 1990;52:115–135. doi: 10.1146/annurev.ph.52.030190.000555. [DOI] [PubMed] [Google Scholar]
- Miichi H., Nagataki S. Effects of pilocarpine, salbutamol, and timolol on aqueous humor formation in cynomolgus monkeys. Invest Ophthalmol Vis Sci. 1983 Sep;24(9):1269–1275. [PubMed] [Google Scholar]
- Mito T., Delamere N. A., Coca-Prados M. Calcium-dependent regulation of cation transport in cultured human nonpigmented ciliary epithelial cells. Am J Physiol. 1993 Mar;264(3 Pt 1):C519–C526. doi: 10.1152/ajpcell.1993.264.3.C519. [DOI] [PubMed] [Google Scholar]
- Okisaka S., Kuwabara T. Selective destruction of the pigmented epithelium in the ciliary body of the eye. Science. 1974 Jun 21;184(4143):1298–1299. doi: 10.1126/science.184.4143.1298. [DOI] [PubMed] [Google Scholar]
- Potter D. E., Burke J. A., Temple J. R. Forskolin suppresses sympathetic neuron function and causes ocular hypotension. Curr Eye Res. 1985 Feb;4(2):87–96. doi: 10.3109/02713688508999973. [DOI] [PubMed] [Google Scholar]
- Reuss L., Segal Y., Altenberg G. Regulation of ion transport across gallbladder epithelium. Annu Rev Physiol. 1991;53:361–373. doi: 10.1146/annurev.ph.53.030191.002045. [DOI] [PubMed] [Google Scholar]
- Schenker H. I., Yablonski M. E., Podos S. M., Linder L. Fluorophotometric study of epinephrine and timolol in human subjects. Arch Ophthalmol. 1981 Jul;99(7):1212–1216. doi: 10.1001/archopht.1981.03930020086007. [DOI] [PubMed] [Google Scholar]
- Sears M. L., Yamada E., Cummins D., Mori N., Mead A., Murakami M. The isolated ciliary bilayer is useful for studies of aqueous humor formation. Trans Am Ophthalmol Soc. 1991;89:131–154. [PMC free article] [PubMed] [Google Scholar]
- Shafiq J., Suzuki S., Itoh T., Kuriyama H. Mechanisms of vasodilation induced by NKH477, a water-soluble forskolin derivative, in smooth muscle of the porcine coronary artery. Circ Res. 1992 Jul;71(1):70–81. doi: 10.1161/01.res.71.1.70. [DOI] [PubMed] [Google Scholar]
- Stetson D. L., Beauwens R., Palmisano J., Mitchell P. P., Steinmetz P. R. A double-membrane model for urinary bicarbonate secretion. Am J Physiol. 1985 Oct;249(4 Pt 2):F546–F552. doi: 10.1152/ajprenal.1985.249.4.F546. [DOI] [PubMed] [Google Scholar]
- Topper J. E., Brubaker R. F. Effects of timolol, epinephrine, and acetazolamide on aqueous flow during sleep. Invest Ophthalmol Vis Sci. 1985 Oct;26(10):1315–1319. [PubMed] [Google Scholar]
- Usukura J., Fain G. L., Bok D. [3H]ouabain localization of Na-K ATPase in the epithelium of rabbit ciliary body pars plicata. Invest Ophthalmol Vis Sci. 1988 Apr;29(4):606–614. [PubMed] [Google Scholar]
- Waitzman M. B., Woods W. D. Some characteristics of an adenyl cyclase preparation from rabbit ciliary process tissue. Exp Eye Res. 1971 Jul;12(1):99–111. doi: 10.1016/0014-4835(71)90134-5. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Zadunaisky J. A. Membrane potentials and intracellular chloride activity in the ciliary body of the shark. Pflugers Arch. 1986;407 (Suppl 2):S112–S115. doi: 10.1007/BF00584939. [DOI] [PubMed] [Google Scholar]
- Yoshitomi T., Gregory D. S. Ocular adrenergic nerves contribute to control of the circadian rhythm of aqueous flow in rabbits. Invest Ophthalmol Vis Sci. 1991 Mar;32(3):523–528. [PubMed] [Google Scholar]
- Yoshitomi T., Horio B., Gregory D. S. Changes in aqueous norepinephrine and cyclic adenosine monophosphate during the circadian cycle in rabbits. Invest Ophthalmol Vis Sci. 1991 Apr;32(5):1609–1613. [PubMed] [Google Scholar]
- Zimmerman T. J., Harbin R., Pett M., Kaufman H. E. Timolol and facility of outflow. Invest Ophthalmol Vis Sci. 1977 Jul;16(7):623–624. [PubMed] [Google Scholar]


