Abstract
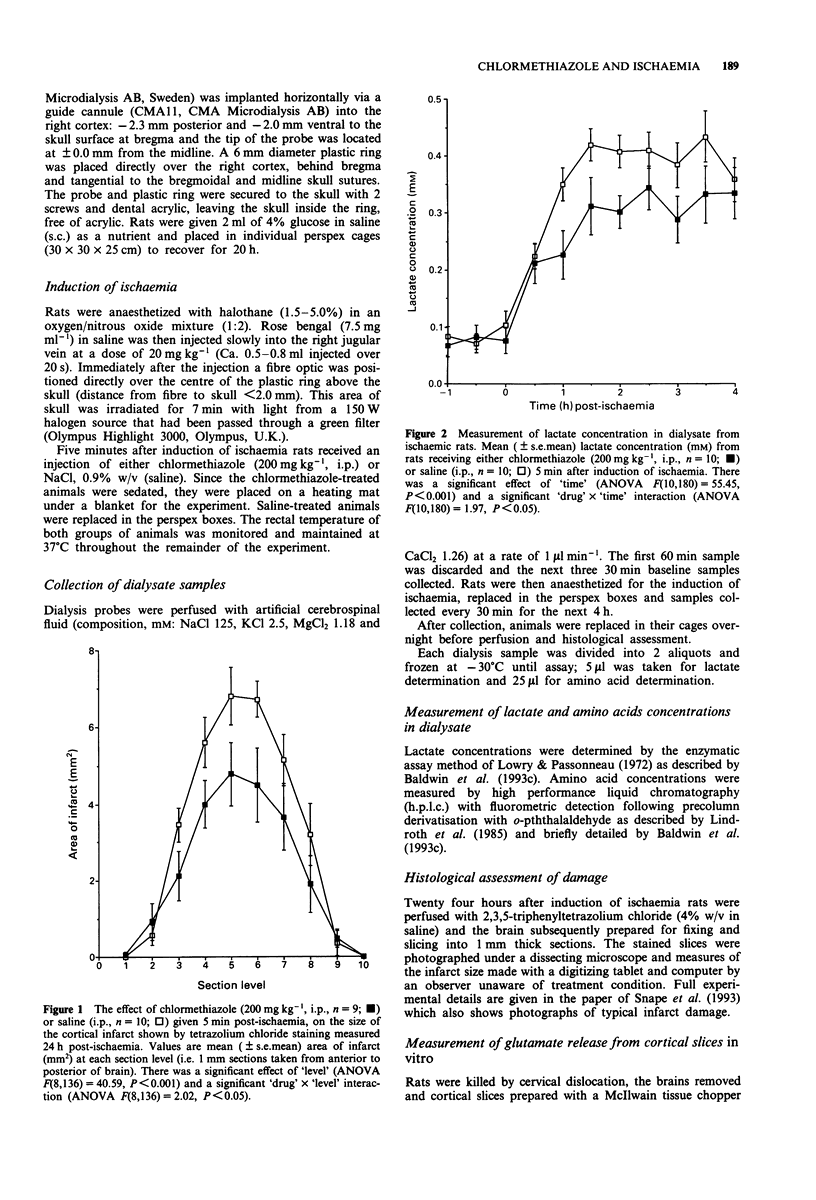
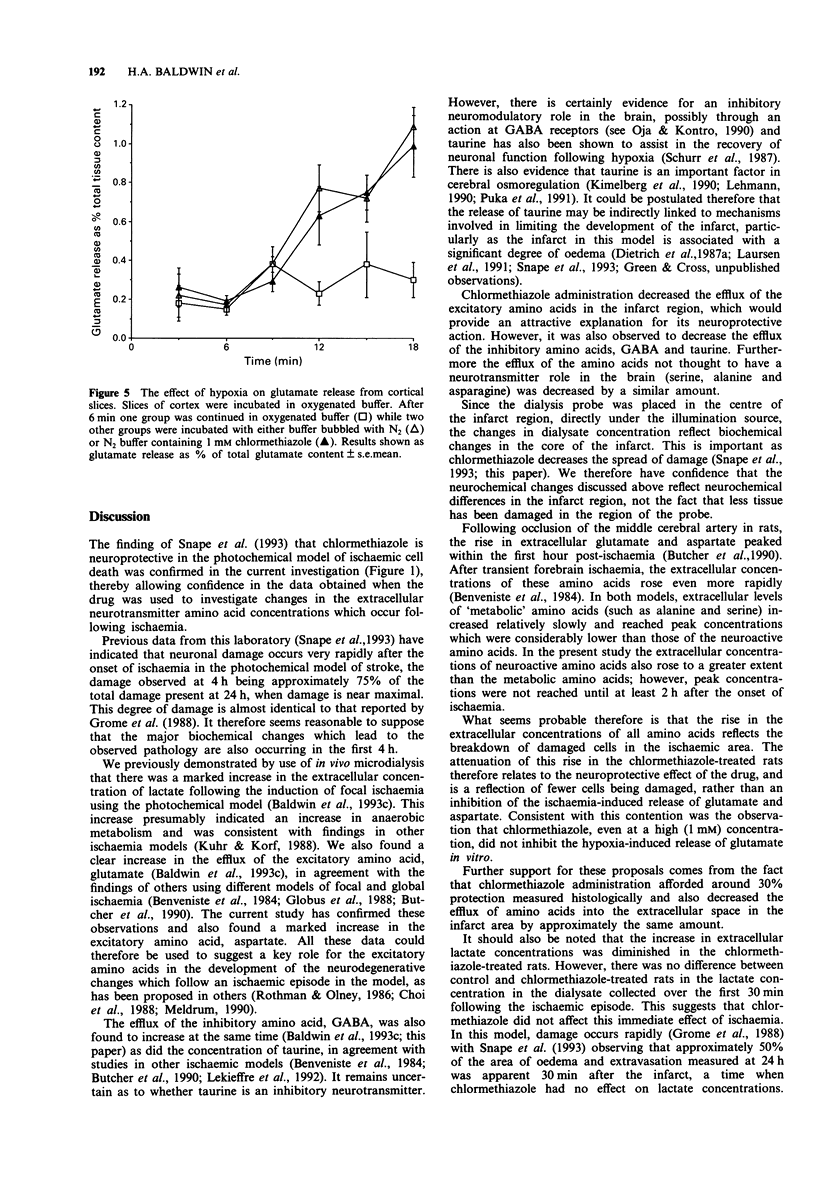
1. In vivo microdialysis has been used to investigate the concentration of various amino acids and lactate in the extracellular fluid of the rat cortex following focal ischaemia, the probe being placed in the core of the infarct area. 2. An ischaemic infarct was produced in the cortex by use of a photochemical dye (Rose Bengal) and light irradiation. There was a marked increase in lactate concentration (300%) over the next 4 h. Substantial increases were also seen in the concentration of the excitatory (glutamate and aspartate), inhibitory (GABA and taurine) and other amino acids (serine, alanine, asparagine). 3. Administration of chlormethiazole (200 mg kg-1, i.p.) 5 min after the onset of ischaemia reduced the ischaemia-induced neurodegeneration by approximately 30%, measured histologically 24 h later. 4. Chlormethiazole (200 mg kg-1, i.p.) administration also reduced the rise in the concentration of lactate and all the amino acids by between 30-60% during the first 4 h after the onset of ischaemia. 5. Analysis of the time course of the amino acid changes suggested that chlormethiazole is not neuroprotective because of the inhibition of excitatory amino acid release but rather that the attenuated rise in the concentration of all the amino acids is reflective of neuroprotection and therefore decreased cell death. 6. This conclusion was supported by the observation that the enhanced efflux of glutamate from slices of cerebral cortex which had been induced by incubation of the slices in an hypoxic medium was unaltered by the presence of a high concentration of chlormethiazole (1 mM) in the medium.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
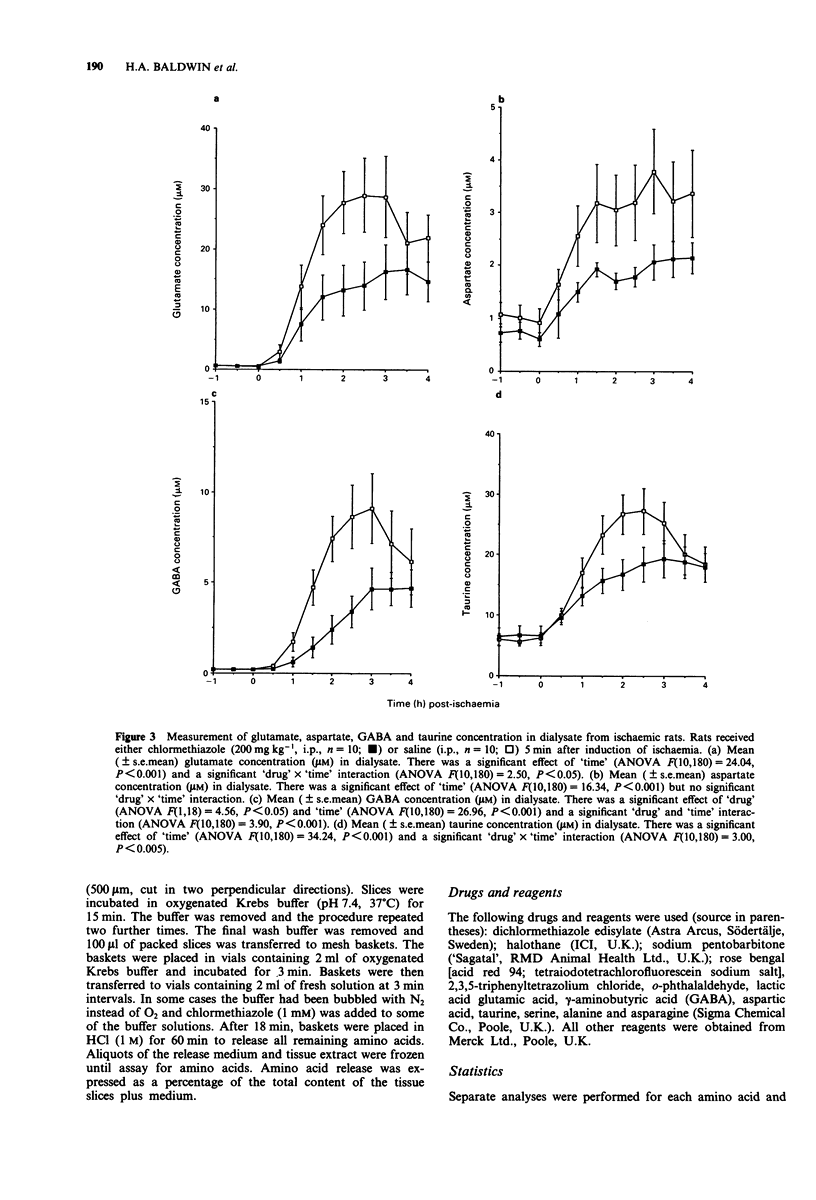
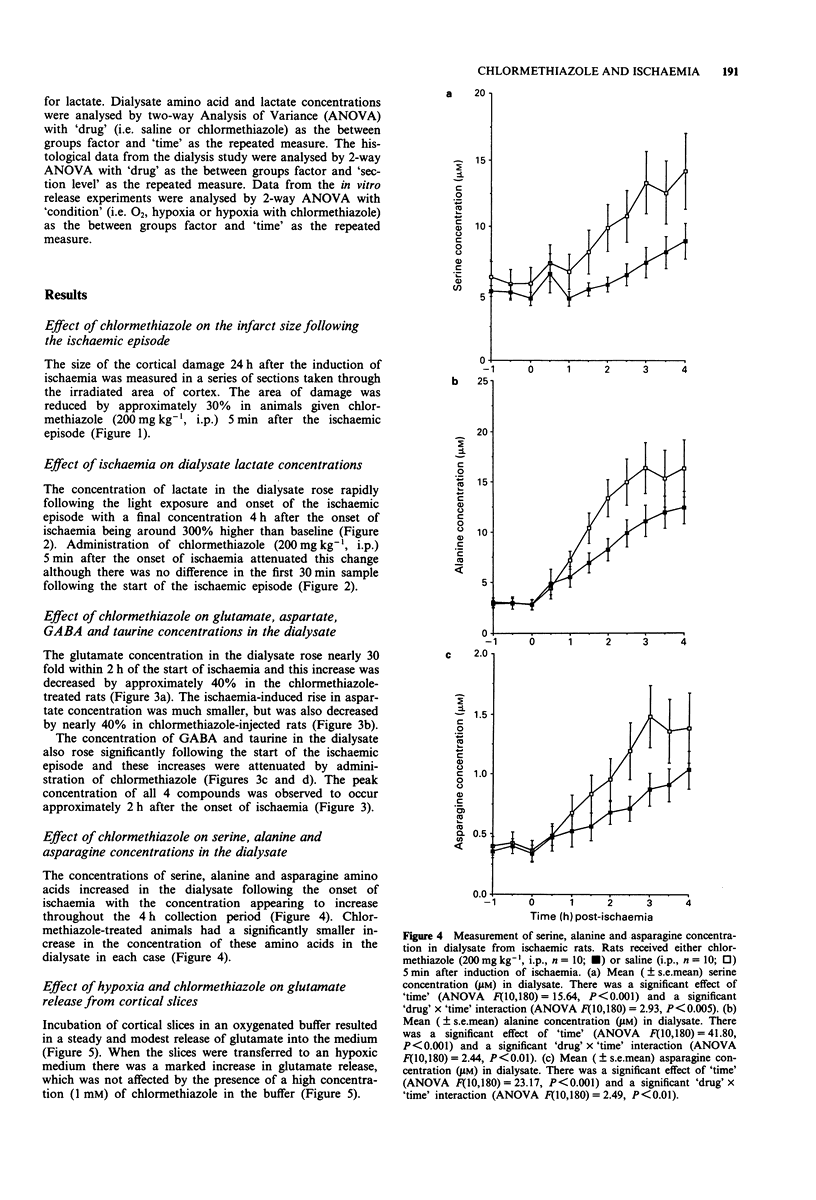


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste H., Drejer J., Schousboe A., Diemer N. H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984 Nov;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Butcher S. P., Bullock R., Graham D. I., McCulloch J. Correlation between amino acid release and neuropathologic outcome in rat brain following middle cerebral artery occlusion. Stroke. 1990 Dec;21(12):1727–1733. doi: 10.1161/01.str.21.12.1727. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Koh J. Y., Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988 Jan;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. J., Jones J. A., Baldwin H. A., Green A. R. Neuroprotective activity of chlormethiazole following transient forebrain ischaemia in the gerbil. Br J Pharmacol. 1991 Oct;104(2):406–411. doi: 10.1111/j.1476-5381.1991.tb12443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ryck M., Van Reempts J., Borgers M., Wauquier A., Janssen P. A. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989 Oct;20(10):1383–1390. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- Dietrich W. D., Busto R., Watson B. D., Scheinberg P., Ginsberg M. D. Photochemically induced cerebral infarction. II. Edema and blood-brain barrier disruption. Acta Neuropathol. 1987;72(4):326–334. doi: 10.1007/BF00687263. [DOI] [PubMed] [Google Scholar]
- Dietrich W. D., Watson B. D., Busto R., Ginsberg M. D., Bethea J. R. Photochemically induced cerebral infarction. I. Early microvascular alterations. Acta Neuropathol. 1987;72(4):315–325. doi: 10.1007/BF00687262. [DOI] [PubMed] [Google Scholar]
- Gill R., Foster A. C., Woodruff G. N. MK-801 is neuroprotective in gerbils when administered during the post-ischaemic period. Neuroscience. 1988 Jun;25(3):847–855. doi: 10.1016/0306-4522(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Gill R., Foster A. C., Woodruff G. N. Systemic administration of MK-801 protects against ischemia-induced hippocampal neurodegeneration in the gerbil. J Neurosci. 1987 Oct;7(10):3343–3349. doi: 10.1523/JNEUROSCI.07-10-03343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R., Nordholm L., Lodge D. The neuroprotective actions of 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline (NBQX) in a rat focal ischaemia model. Brain Res. 1992 May 15;580(1-2):35–43. doi: 10.1016/0006-8993(92)90924-x. [DOI] [PubMed] [Google Scholar]
- Globus M. Y., Busto R., Dietrich W. D., Martinez E., Valdes I., Ginsberg M. D. Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and gamma-aminobutyric acid studied by intracerebral microdialysis. J Neurochem. 1988 Nov;51(5):1455–1464. doi: 10.1111/j.1471-4159.1988.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Grome J. J., Gojowczyk G., Hofmann W., Graham D. I. Quantitation of photochemically induced focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1988 Feb;8(1):89–95. doi: 10.1038/jcbfm.1988.11. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Goderie S. K., Higman S., Pang S., Waniewski R. A. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990 May;10(5):1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhr W. G., Korf J. Extracellular lactic acid as an indicator of brain metabolism: continuous on-line measurement in conscious, freely moving rats with intrastriatal dialysis. J Cereb Blood Flow Metab. 1988 Feb;8(1):130–137. doi: 10.1038/jcbfm.1988.17. [DOI] [PubMed] [Google Scholar]
- Lehmann A. Derangements in cerebral osmohomeostasis: a common denominator for stimulation of taurine and phosphoethanolamine release. Prog Clin Biol Res. 1990;351:337–347. [PubMed] [Google Scholar]
- Lekieffre D., Callebert J., Plotkine M., Boulu R. G. Concomitant increases in the extracellular concentrations of excitatory and inhibitory amino acids in the rat hippocampus during forebrain ischemia. Neurosci Lett. 1992 Mar 16;137(1):78–82. doi: 10.1016/0304-3940(92)90303-o. [DOI] [PubMed] [Google Scholar]
- Meldrum B. Protection against ischaemic neuronal damage by drugs acting on excitatory neurotransmission. Cerebrovasc Brain Metab Rev. 1990 Spring;2(1):27–57. [PubMed] [Google Scholar]
- Oja S. S., Kontro P. Neuromodulatory and trophic actions of taurine. Prog Clin Biol Res. 1990;351:69–76. [PubMed] [Google Scholar]
- Park C. K., Nehls D. G., Graham D. I., Teasdale G. M., McCulloch J. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann Neurol. 1988 Oct;24(4):543–551. doi: 10.1002/ana.410240411. [DOI] [PubMed] [Google Scholar]
- Puka M., Sundell K., Lazarewicz J. W., Lehmann A. Species differences in cerebral taurine concentrations correlate with brain water content. Brain Res. 1991 May 10;548(1-2):267–272. doi: 10.1016/0006-8993(91)91131-j. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Schurr A., Tseng M. T., West C. A., Rigor B. M. Taurine improves the recovery of neuronal function following cerebral hypoxia: an in vitro study. Life Sci. 1987 May 25;40(21):2059–2066. doi: 10.1016/0024-3205(87)90098-1. [DOI] [PubMed] [Google Scholar]
- Sheardown M. J., Nielsen E. O., Hansen A. J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Snape M. F., Baldwin H. A., Cross A. J., Green A. R. The effects of chlormethiazole and nimodipine on cortical infarct area after focal cerebral ischaemia in the rat. Neuroscience. 1993 Apr;53(3):837–844. doi: 10.1016/0306-4522(93)90628-s. [DOI] [PubMed] [Google Scholar]
- Watson B. D., Dietrich W. D., Busto R., Wachtel M. S., Ginsberg M. D. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985 May;17(5):497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]


