Abstract
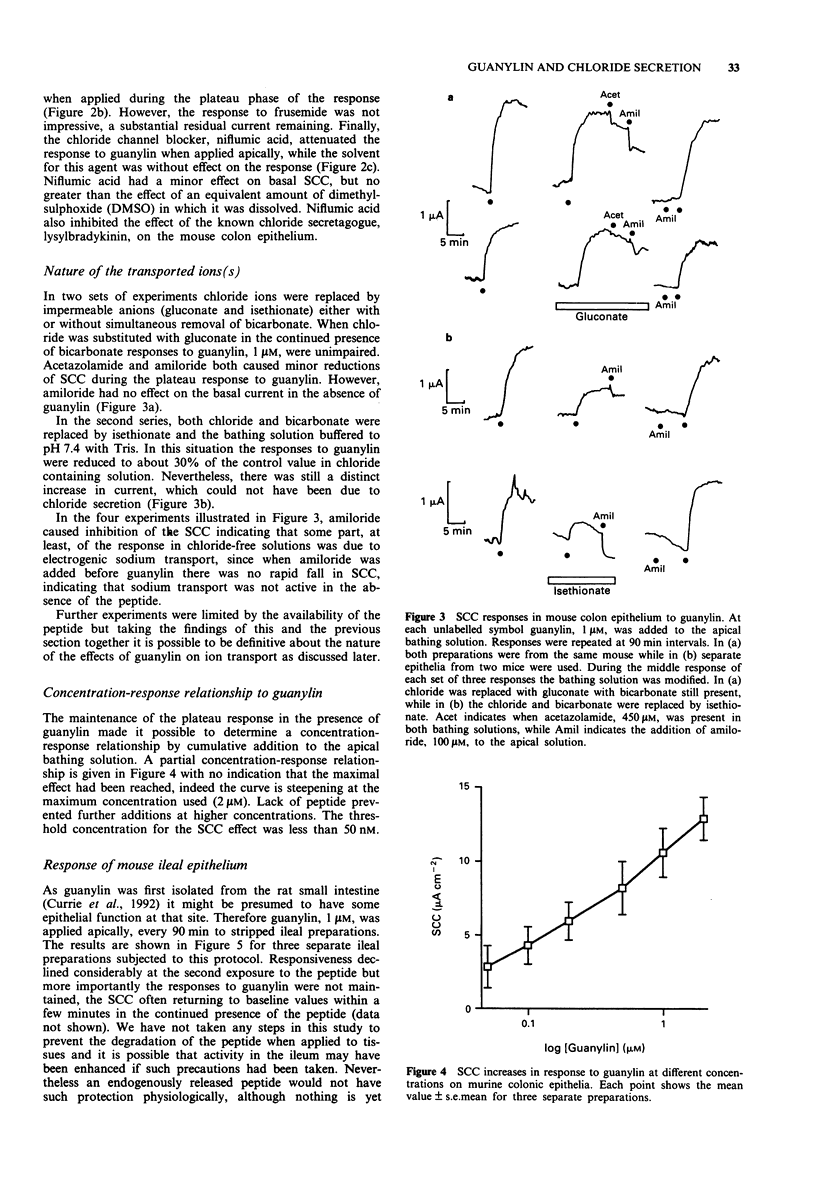
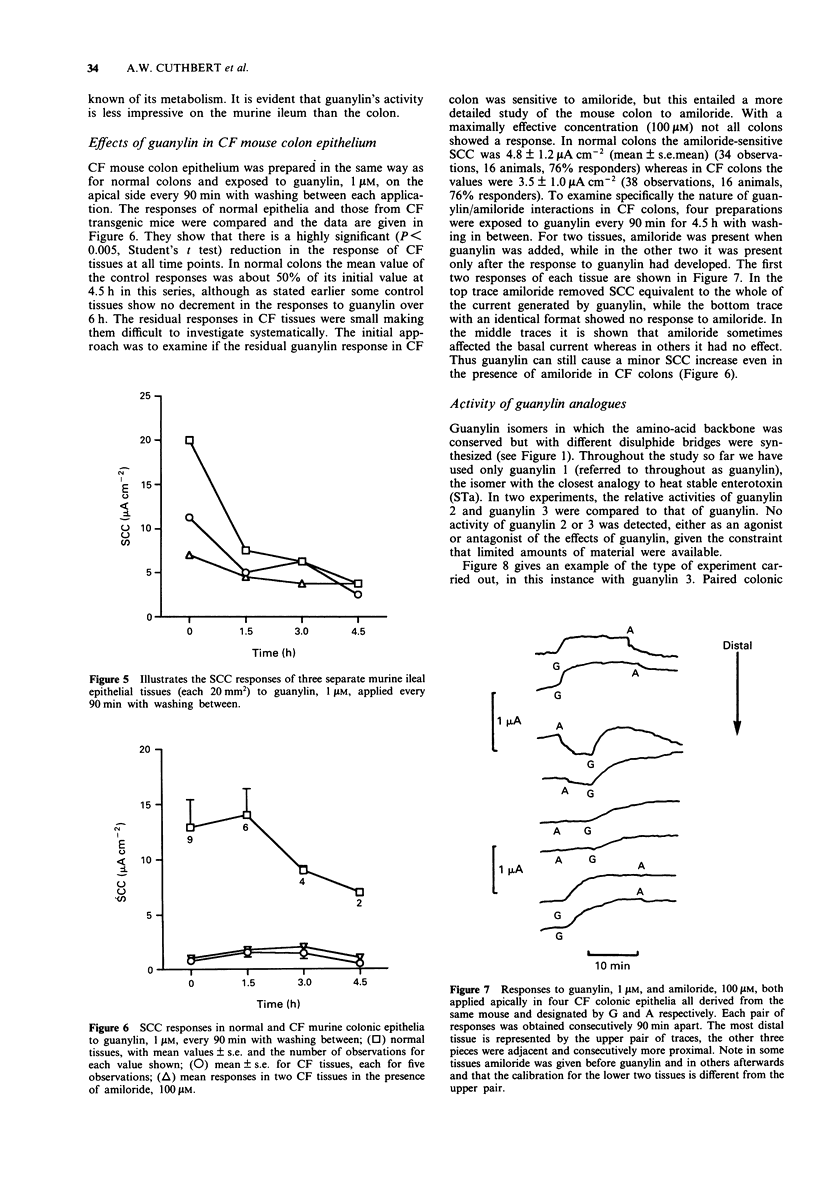
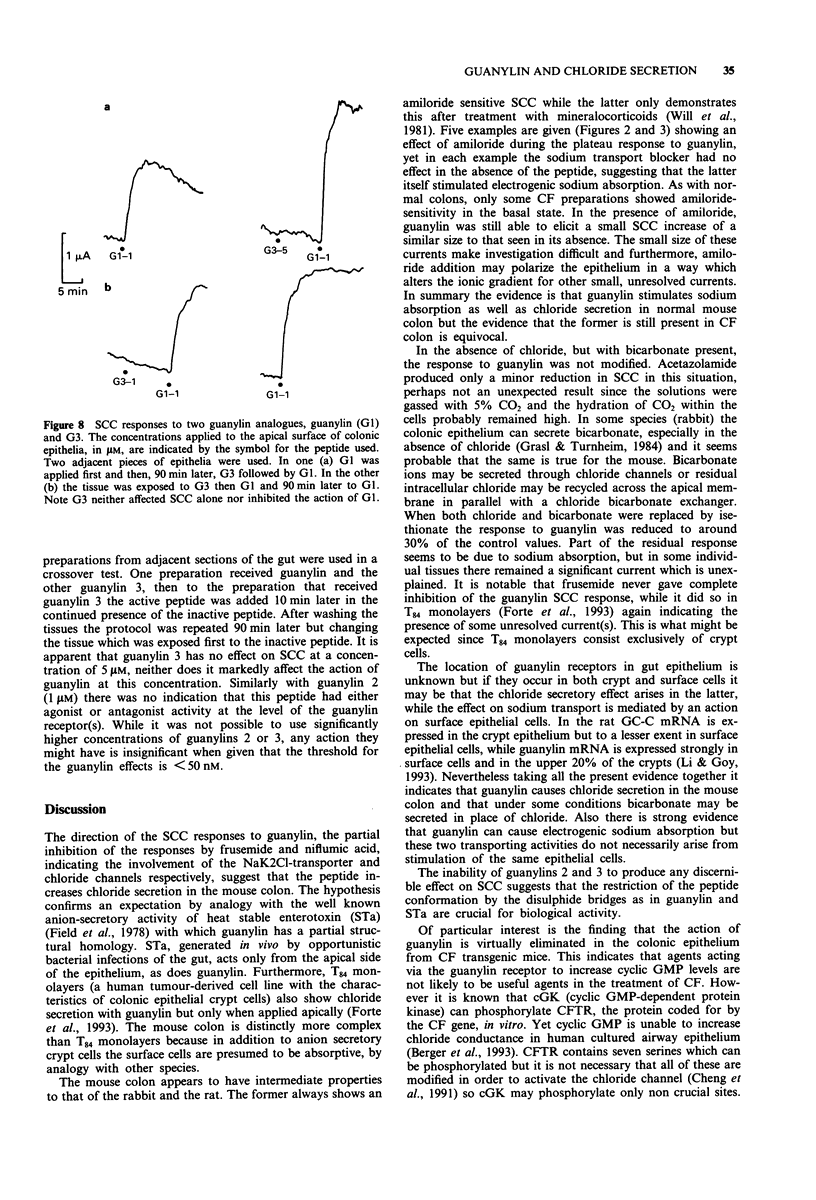
1. Guanylin, a 15 amino acid endogenous gut peptide, increased the short circuit current (SCC) in the epithelium of the mouse colon, but only when applied to the apical and not the basolateral surface. 2. By use of selective blockers of epithelial ion transport and modification of the bathing solution, it was concluded that guanylin increased electrogenic chloride secretion but also had a minor effect on electrogenic sodium absorption. In addition there were small residual currents which remained unresolved. 3. The threshold concentration of guanylin causing a SCC increase was less than 50 nM, but at concentrations 40 times greater no indication of a maximally effective concentration was found. 4. Two guanylin isomers with the same amino acid sequence but with the disulphide bridges joined in an alternate fashion showed no activity. Thus only guanylin with the greatest structural homology to heat stable enterotoxin (STa) showed biological activity. 5. The action of guanylin was virtually eliminated in colonic epithelia from transgenic cystic fibrosis (CF) mice. As these animals lack the chloride channel coded by the CF gene sequence, it is likely that the final effector process in murine colonic epithelia involves the CFTR (cystic fibrosis transmembrane conductance regulator) chloride channel. 6. Opportunistic infections of the gut generating STa lead to diarrhoeal conditions via an action of the toxin on apical guanylin receptors. Thus, as discussed, the CF heterozygote may have a genetic advantage in this circumstance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger H. A., Travis S. M., Welsh M. J. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by specific protein kinases and protein phosphatases. J Biol Chem. 1993 Jan 25;268(3):2037–2047. [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Rich D. P., Marshall J., Gregory R. J., Welsh M. J., Smith A. E. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991 Sep 6;66(5):1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M., Flotte T., Afione S., Solow R., Zeitlin P. L., Carter B. J., Guggino W. B. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature. 1992 Aug 13;358(6387):581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Semrad C. E. Toxigenic diarrheas, congenital diarrheas, and cystic fibrosis: disorders of intestinal ion transport. Annu Rev Physiol. 1993;55:631–655. doi: 10.1146/annurev.ph.55.030193.003215. [DOI] [PubMed] [Google Scholar]
- Forte L. R., Eber S. L., Turner J. T., Freeman R. H., Fok K. F., Currie M. G. Guanylin stimulation of Cl- secretion in human intestinal T84 cells via cyclic guanosine monophosphate. J Clin Invest. 1993 Jun;91(6):2423–2428. doi: 10.1172/JCI116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte L. R., Thorne P. K., Eber S. L., Krause W. J., Freeman R. H., Francis S. H., Corbin J. D. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992 Sep;263(3 Pt 1):C607–C615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- Grasl M., Turnheim K. Stimulation of electrolyte secretion in rabbit colon by adenosine. J Physiol. 1984 Jan;346:93–110. doi: 10.1113/jphysiol.1984.sp015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. C. Cystic fibrosis and chloride-secreting diarrhoea. Nature. 1988 Jun 23;333(6175):711–711. doi: 10.1038/333711c0. [DOI] [PubMed] [Google Scholar]
- Li Z., Goy M. F. Peptide-regulated guanylate cyclase pathways in rat colon: in situ localization of GCA, GCC, and guanylin mRNA. Am J Physiol. 1993 Aug;265(2 Pt 1):G394–G402. doi: 10.1152/ajpgi.1993.265.2.G394. [DOI] [PubMed] [Google Scholar]
- O'Loughlin E. V., Hunt D. M., Gaskin K. J., Stiel D., Bruzuszcak I. M., Martin H. C., Bambach C., Smith R. Abnormal epithelial transport in cystic fibrosis jejunum. Am J Physiol. 1991 May;260(5 Pt 1):G758–G763. doi: 10.1152/ajpgi.1991.260.5.G758. [DOI] [PubMed] [Google Scholar]
- Ratcliff R., Evans M. J., Cuthbert A. W., MacVinish L. J., Foster D., Anderson J. R., Colledge W. H. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993 May;4(1):35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Kato J., Currie M. G. Rat guanylin cDNA: characterization of the precursor of an endogenous activator of intestinal guanylate cyclase. Biochem Biophys Res Commun. 1992 Jun 30;185(3):812–817. doi: 10.1016/0006-291x(92)91699-q. [DOI] [PubMed] [Google Scholar]
- Will P. C., DeLisle R. C., Cortright R. N., Hopfer U. Induction of amiloride-sensitive sodium transport in the intestines by adrenal steroids. Ann N Y Acad Sci. 1981;372:64–78. doi: 10.1111/j.1749-6632.1981.tb15458.x. [DOI] [PubMed] [Google Scholar]
- Wong S. K., Garbers D. L. Receptor guanylyl cyclases. J Clin Invest. 1992 Aug;90(2):299–305. doi: 10.1172/JCI115862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge H. R., van den Berghe N., Tilly B. C., Kansen M., Bijman J. (Dys)regulation of epithelial chloride channels. Biochem Soc Trans. 1989 Oct;17(5):816–818. doi: 10.1042/bst0170816. [DOI] [PubMed] [Google Scholar]


