Abstract
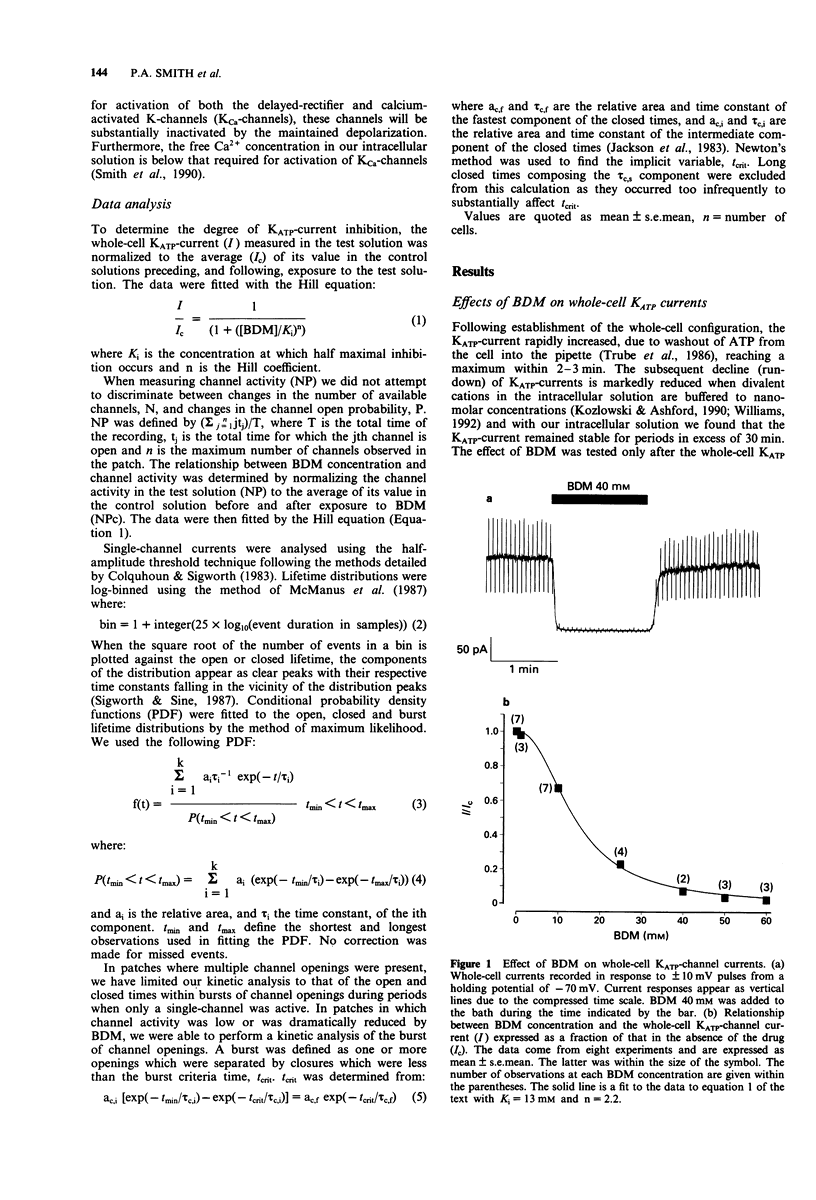
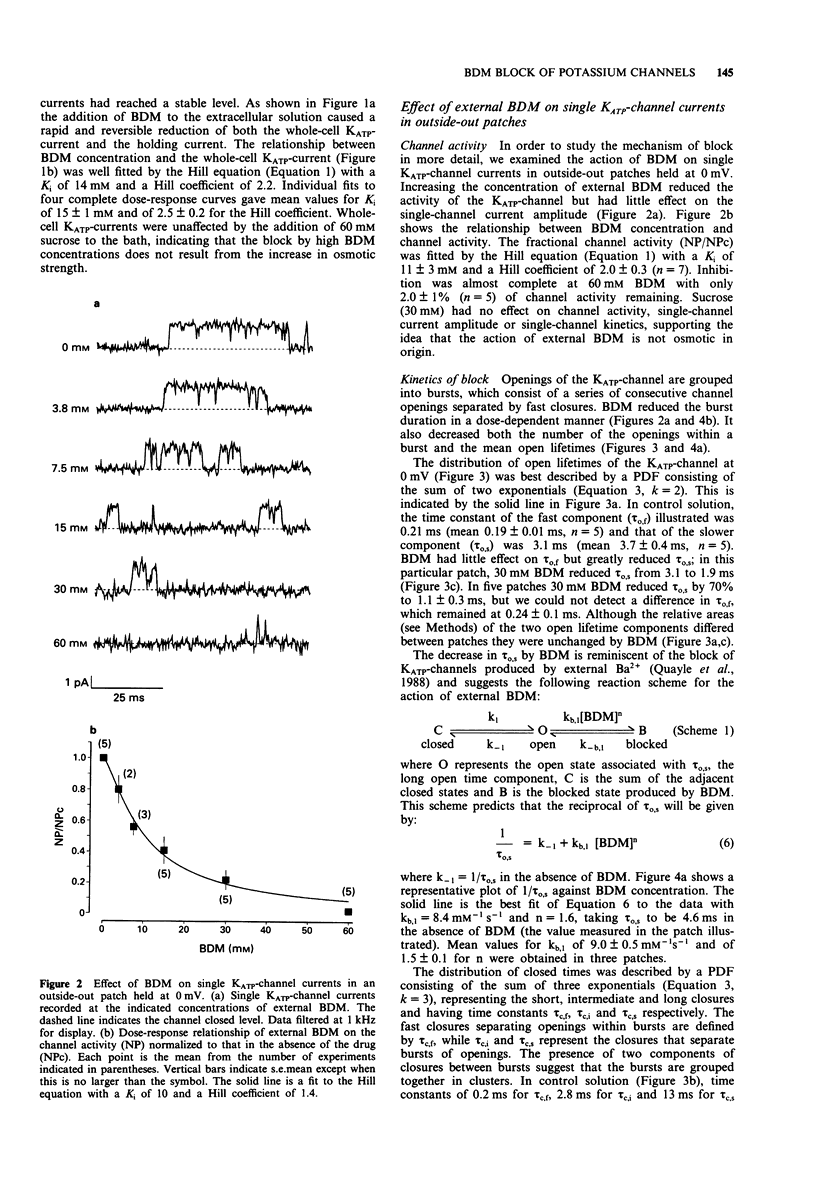
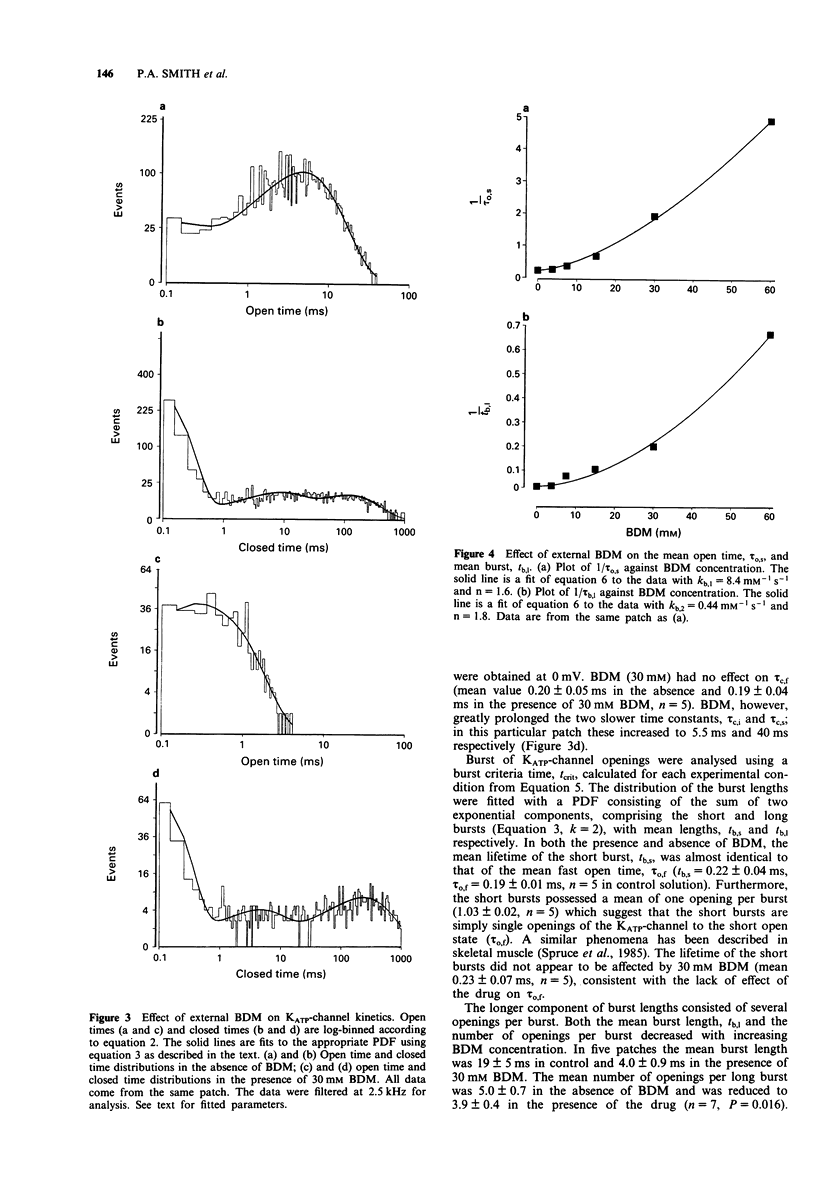
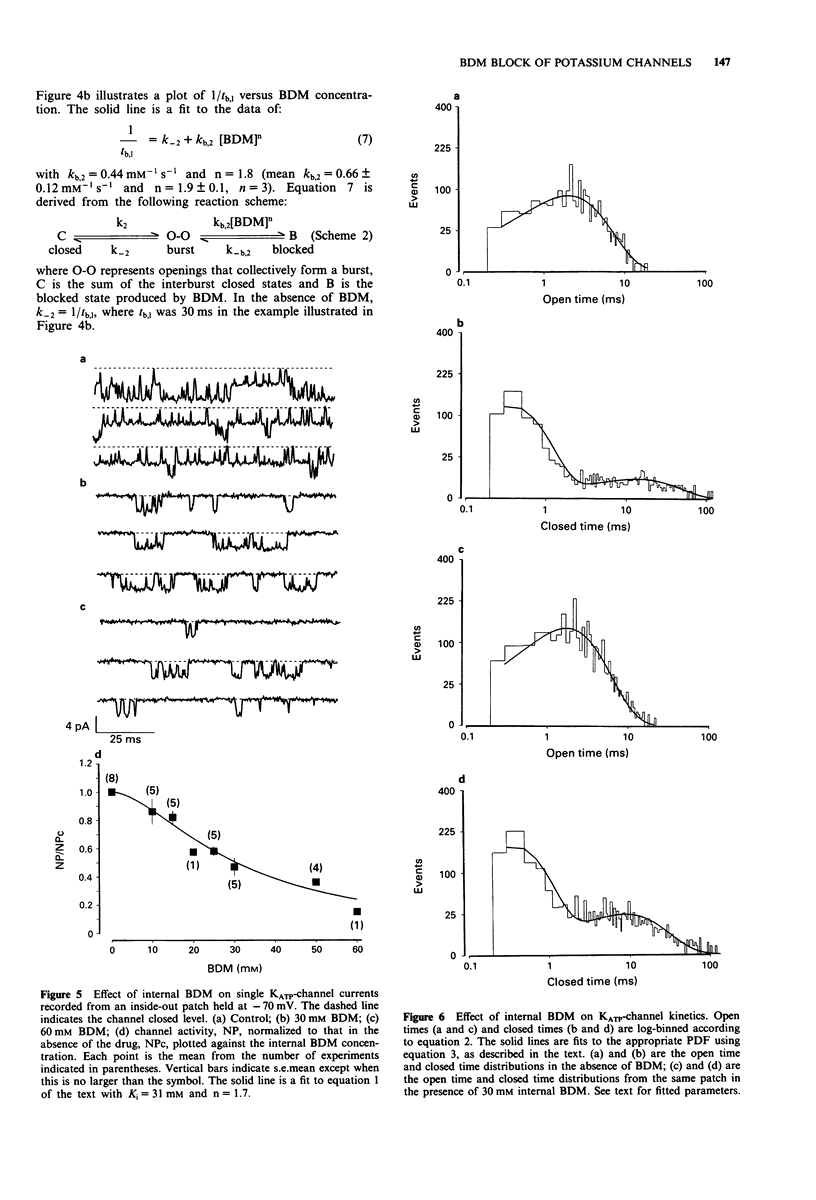
1. The patch-clamp technique has been used to examine the action of the chemical phosphatase 2,3-butanedione monoxime (BDM) on ATP-sensitive K+ channels (KATP-channels) from mouse isolated pancreatic beta-cells in the absence of ATP and Mg2+. 2. BDM reversibly inhibited whole-cell KATP-currents with a concentration for half maximal inhibition (K(i)) of 15 +/- 1 mM and a Hill coefficient (n) of 2.5 +/- 0.2 (n = 4). 3. In outside-out patches, external BDM reversibly reduced the activity of single KATP-channels with an affinity similar to that observed in whole-cell recordings (K(i) = 11 +/- 3 mM, n = 2.0 +/- 0.3, n = 7). In inside-out patches, internally applied BDM also reversibly blocked the activity of KATP-channels (K(i) = 31 +/- 2 mM, n = 2.2 +/- 0.4, n = 8). In both excised patch configurations, BDM decreased the mean open life-time and the burst duration, thereby producing a decrease in the channel open probability. The drug had no effect on the short intraburst closed times. 4. BDM had no effect on the single-channel current amplitude. 5. The results suggest that BDM blocks the KATP-channel directly, by mechanisms independent of channel dephosphorylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokvist K., Rorsman P., Smith P. A. Effects of external tetraethylammonium ions and quinine on delayed rectifying K+ channels in mouse pancreatic beta-cells. J Physiol. 1990 Apr;423:311–325. doi: 10.1113/jphysiol.1990.sp018024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. The effect of oximes on the dihydropyridine-sensitive Ca current of isolated guinea-pig ventricular myocytes. Pflugers Arch. 1993 Jan;422(4):325–331. doi: 10.1007/BF00374287. [DOI] [PubMed] [Google Scholar]
- Coulombe A., Lefevre I. A., Deroubaix E., Thuringer D., Coraboeuf E. Effect of 2,3-butanedione 2-monoxime on slow inward and transient outward currents in rat ventricular myocytes. J Mol Cell Cardiol. 1990 Aug;22(8):921–932. doi: 10.1016/0022-2828(90)90123-j. [DOI] [PubMed] [Google Scholar]
- Davies N. W., Spruce A. E., Standen N. B., Stanfield P. R. Multiple blocking mechanisms of ATP-sensitive potassium channels of frog skeletal muscle by tetraethylammonium ions. J Physiol. 1989 Jun;413:31–48. doi: 10.1113/jphysiol.1989.sp017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis K. D., Gee W. M., Hammoud A., McDaniel M. L., Falke L. C., Misler S. Effects of sulfonamides on a metabolite-regulated ATPi-sensitive K+ channel in rat pancreatic B-cells. Am J Physiol. 1989 Dec;257(6 Pt 1):C1119–C1127. doi: 10.1152/ajpcell.1989.257.6.C1119. [DOI] [PubMed] [Google Scholar]
- Huang G. J., McArdle J. J. Novel suppression of an L-type calcium channel in neurones of murine dorsal root ganglia by 2,3-butanedione monoxime. J Physiol. 1992 Feb;447:257–274. doi: 10.1113/jphysiol.1992.sp019001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Lopatin A. N., Nichols C. G. 2,3-Butanedione monoxime (BDM) inhibition of delayed rectifier DRK1 (Kv2.1) potassium channels expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1993 May;265(2):1011–1016. [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985 Dec;405(4):305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Bokvist K., Arkhammar P., Berggren P. O., Rorsman P. Delayed rectifying and calcium-activated K+ channels and their significance for action potential repolarization in mouse pancreatic beta-cells. J Gen Physiol. 1990 Jun;95(6):1041–1059. doi: 10.1085/jgp.95.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985 Aug 22;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Zilberter Y., Burnashev N., Papin A., Portnov V., Khodorov B. Gating kinetics of ATP-sensitive single potassium channels in myocardial cells depends on electromotive force. Pflugers Arch. 1988 May;411(5):584–589. doi: 10.1007/BF00582382. [DOI] [PubMed] [Google Scholar]


