Abstract
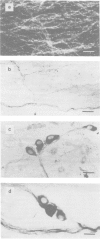
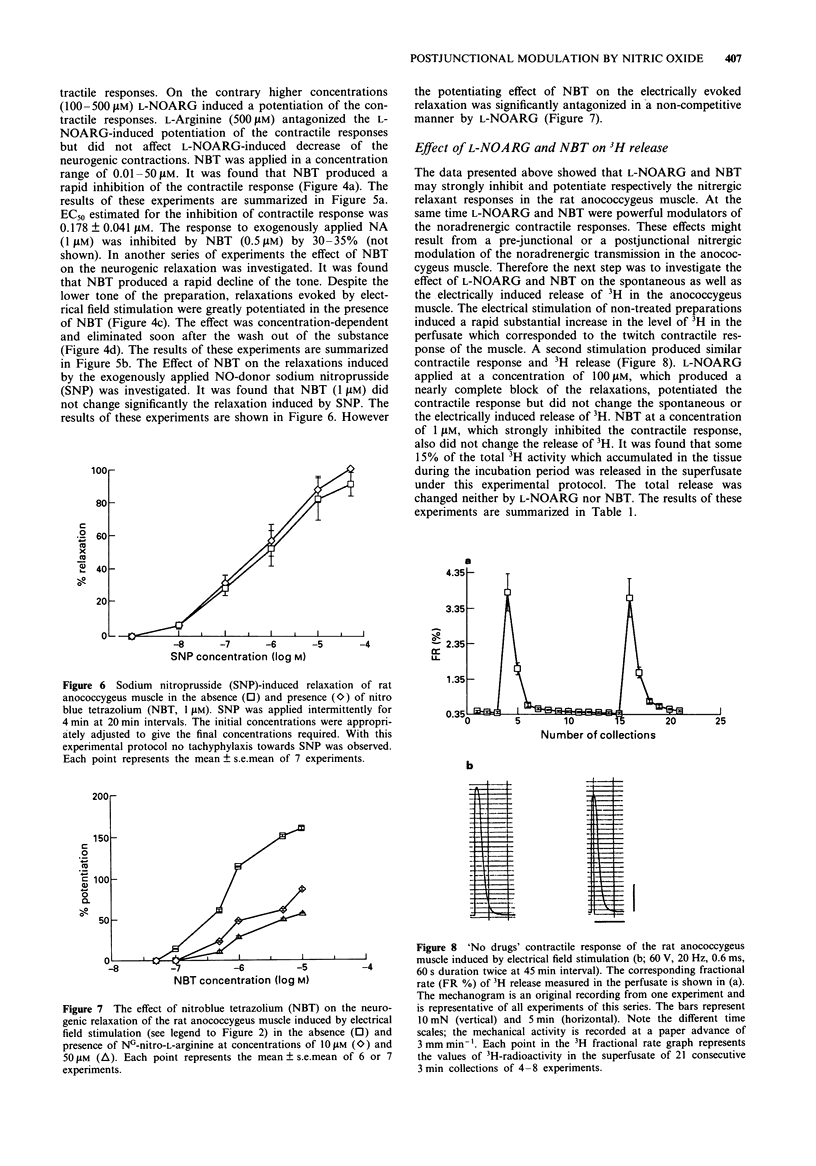
1. The distribution of NADPH-diaphorase positive and catecholamine-containing nerve structures, and functional noradrenergic-nitrergic interactions, were studied in the rat anococcygeus muscle. 2. The morphological findings demonstrated NADPH-diaphorase positive neurons mostly as aggregates in intramural ganglia, nerve tracts and few single nerve fibres forming plexus-like structures. 3. The nitric oxide synthase inhibitor NG-nitro-L-arginine (L-NOARG) inhibited concentration-dependently the nitrergic relaxation, an effect reversed by L-arginine. The drug had dual effects on noradrenergic contractile responses: at lower concentrations (0.1-10 microM) it decreased the amplitude of contractions and this was not affected by L-arginine; higher concentrations (50-500 microM) potentiated the contractions, an effect that was prevented by L-arginine. 4. The electron acceptor, nitro blue tetrazolium (NBT) produced a rapid inhibition of the noradrenergic contractile responses (EC50 0.178 +/- 0.041 microM). The drug decreased the tone of the preparations. However, it potentiated concentration-dependently the nitrergic relaxations. 5. NBT (1 microM) had no significant effect on the relaxations induced by exogenously applied nitric oxide (NO)-donor sodium nitroprusside (SNP, 0.01-50 microM). However, the effect of NBT (0.1-10 microM) on the electrically induced relaxation was significantly decreased by L-NOARG (10 and 50 microM). The inhibition was of a non-competitive type. 6. Neither L-NOARG (100 microM) nor NBT (1 microM) had any effect on the spontaneous or electrically-induced release of 3H-radioactivity from the tissues preincubated in [3H]-noradrenaline. 7. It is concluded that L-arginine-NO pathway can modulate noradrenergic transmission in the rat anococcygeus muscle at postjunctional, but not prejunctional site(s).
Full text
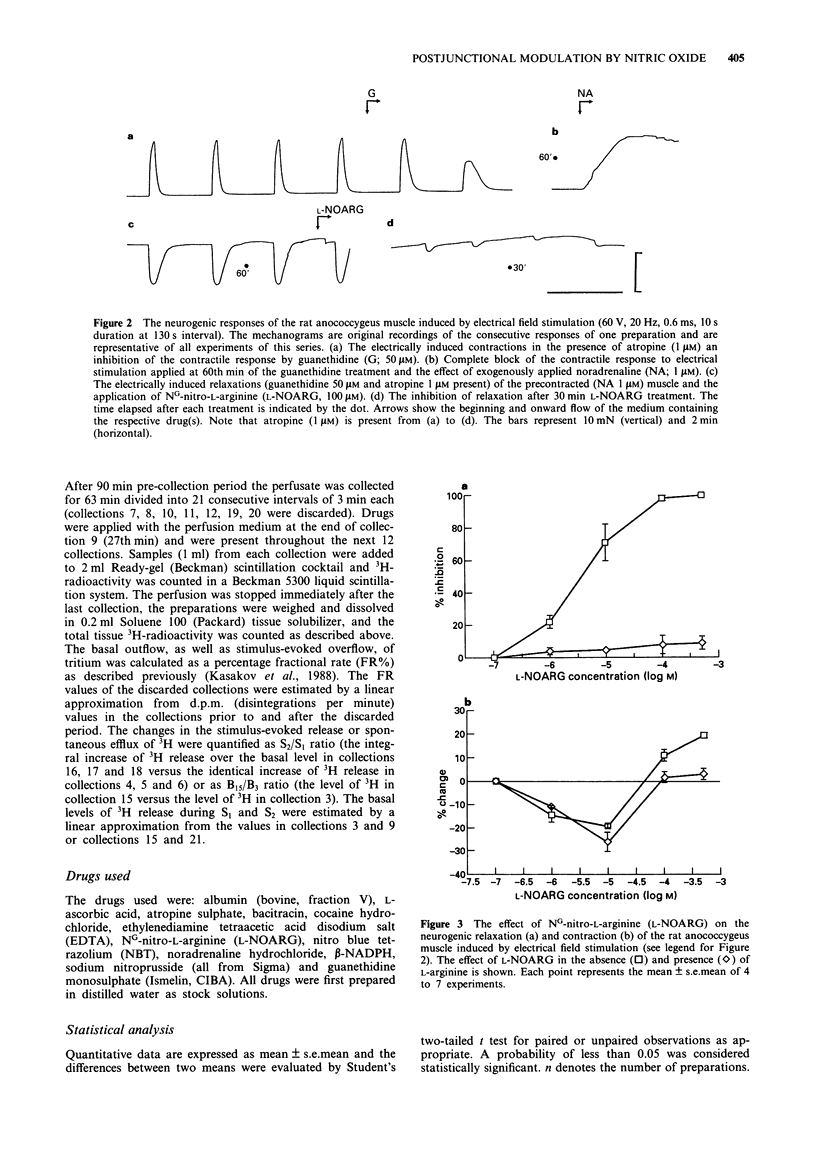
PDF
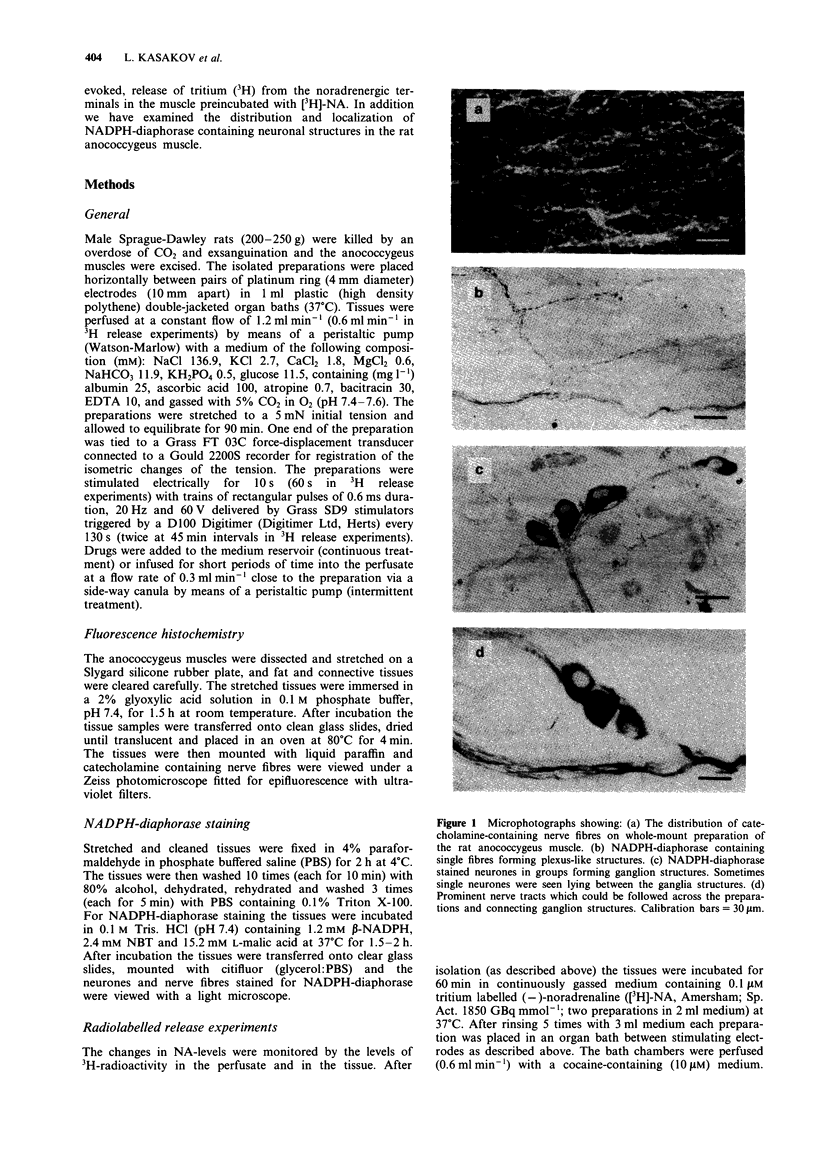
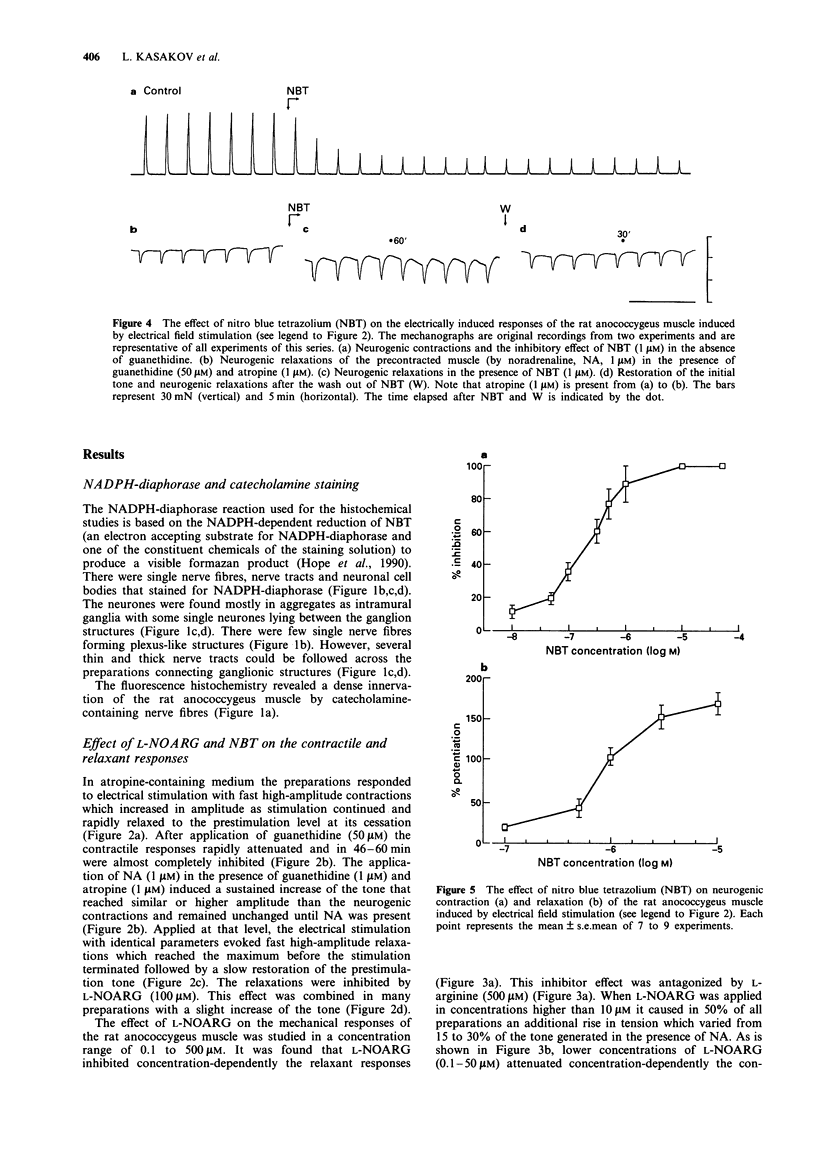



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer S. L., Hampl V. NG-monomethyl-L-arginine causes nitric oxide synthesis in isolated arterial rings: trouble in paradise. Biochem Biophys Res Commun. 1992 Oct 30;188(2):590–596. doi: 10.1016/0006-291x(92)91097-a. [DOI] [PubMed] [Google Scholar]
- Arnold W. P., Mittal C. K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belai A., Schmidt H. H., Hoyle C. H., Hassall C. J., Saffrey M. J., Moss J., Förstermann U., Murad F., Burnstock G. Colocalization of nitric oxide synthase and NADPH-diaphorase in the myenteric plexus of the rat gut. Neurosci Lett. 1992 Aug 31;143(1-2):60–64. doi: 10.1016/0304-3940(92)90233-w. [DOI] [PubMed] [Google Scholar]
- Belvisi M. G., Stretton D., Barnes P. J. Nitric oxide as an endogenous modulator of cholinergic neurotransmission in guinea-pig airways. Eur J Pharmacol. 1991 Jun 6;198(2-3):219–221. doi: 10.1016/0014-2999(91)90626-2. [DOI] [PubMed] [Google Scholar]
- Brave S. R., Bhat S., Hobbs A. J., Tucker J. F., Gibson A. The influence of L-NG-nitro-arginine on sympathetic nerve induced contraction and noradrenaline release in the rat isolated anococcygeus muscle. J Auton Pharmacol. 1993 Jun;13(3):219–225. doi: 10.1111/j.1474-8673.1993.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Glatt C. E., Hwang P. M., Fotuhi M., Dawson T. M., Snyder S. H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991 Oct;7(4):615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Bucher B., Ouedraogo S., Tschöpl M., Paya D., Stoclet J. C. Role of the L-arginine-NO pathway and of cyclic GMP in electrical field-induced noradrenaline release and vasoconstriction in the rat tail artery. Br J Pharmacol. 1992 Dec;107(4):976–982. doi: 10.1111/j.1476-5381.1992.tb13394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R. Evidence for purinergic innervation of the anococcygeus muscle. Br J Pharmacol. 1978 Sep;64(1):13–20. doi: 10.1111/j.1476-5381.1978.tb08635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederqvist B., Wiklund N. P., Persson M. G., Gustafsson L. E. Modulation of neuroeffector transmission in the guinea pig pulmonary artery by endogenous nitric oxide. Neurosci Lett. 1991 Jun 10;127(1):67–69. doi: 10.1016/0304-3940(91)90896-2. [DOI] [PubMed] [Google Scholar]
- Cohen R. A., Weisbrod R. M. Endothelium inhibits norepinephrine release from adrenergic nerves of rabbit carotid artery. Am J Physiol. 1988 May;254(5 Pt 2):H871–H878. doi: 10.1152/ajpheart.1988.254.5.H871. [DOI] [PubMed] [Google Scholar]
- Dail W. G., Galloway B., Bordegaray J. NADPH diaphorase innervation of the rat anococcygeus and retractor penis muscles. Neurosci Lett. 1993 Sep 17;160(1):17–20. doi: 10.1016/0304-3940(93)90906-2. [DOI] [PubMed] [Google Scholar]
- Davisson R. L., Walton T. M., Johnson A. K., Lewis S. J. Cardiovascular effects produced by systemic injections of nitro blue tetrazolium in the rat. Eur J Pharmacol. 1993 Sep 7;241(1):135–137. doi: 10.1016/0014-2999(93)90945-e. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. S., Diecke F. P., Peevy K., Tanaka T. P. Release of norepinephrine from adrenergic nerve endings of blood vessels is modulated by endothelium-derived relaxing factor. Am J Hypertens. 1990 Mar;3(3):211–218. doi: 10.1093/ajh/3.3.211. [DOI] [PubMed] [Google Scholar]
- Greenberg S., Diecke F. P., Peevy K., Tanaka T. P. The endothelium modulates adrenergic neurotransmission to canine pulmonary arteries and veins. Eur J Pharmacol. 1989 Mar 14;162(1):67–80. doi: 10.1016/0014-2999(89)90605-5. [DOI] [PubMed] [Google Scholar]
- Gustafsson L. E., Wiklund C. U., Wiklund N. P., Persson M. G., Moncada S. Modulation of autonomic neuroeffector transmission by nitric oxide in guinea pig ileum. Biochem Biophys Res Commun. 1990 Nov 30;173(1):106–110. doi: 10.1016/s0006-291x(05)81028-9. [DOI] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Ellis J., Kirkpatrick K., Milner P., Burnstock G. Direct evidence for concomitant release of noradrenaline, adenosine 5'-triphosphate and neuropeptide Y from sympathetic nerve supplying the guinea-pig vas deferens. J Auton Nerv Syst. 1988 Feb;22(1):75–82. doi: 10.1016/0165-1838(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Förstermann U., Warner T. D., Pollock J. S., Schmidt H. H., Heller M., Murad F. Endothelial cells have a particulate enzyme system responsible for EDRF formation: measurement by vascular relaxation. Biochem Biophys Res Commun. 1991 May 15;176(3):1417–1423. doi: 10.1016/0006-291x(91)90444-c. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Sheng H., Förstermann U., Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992 Mar;262(3 Pt 1):G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Pollock J. S., Nakane M., Gorsky L. D., Förstermann U., Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Mechanism of neurally induced monkey mesenteric artery relaxation and contraction. Hypertension. 1992 Feb;19(2):161–166. doi: 10.1161/01.hyp.19.2.161. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Modification by L-NG-monomethyl arginine (L-NMMA) of the response to nerve stimulation in isolated dog mesenteric and cerebral arteries. Jpn J Pharmacol. 1990 Jan;52(1):170–173. doi: 10.1254/jjp.52.170. [DOI] [PubMed] [Google Scholar]
- Wiklund N. P., Leone A. M., Gustafsson L. E., Moncada S. Release of nitric oxide evoked by nerve stimulation in guinea-pig intestine. Neuroscience. 1993 Apr;53(3):607–611. doi: 10.1016/0306-4522(93)90609-j. [DOI] [PubMed] [Google Scholar]