Abstract
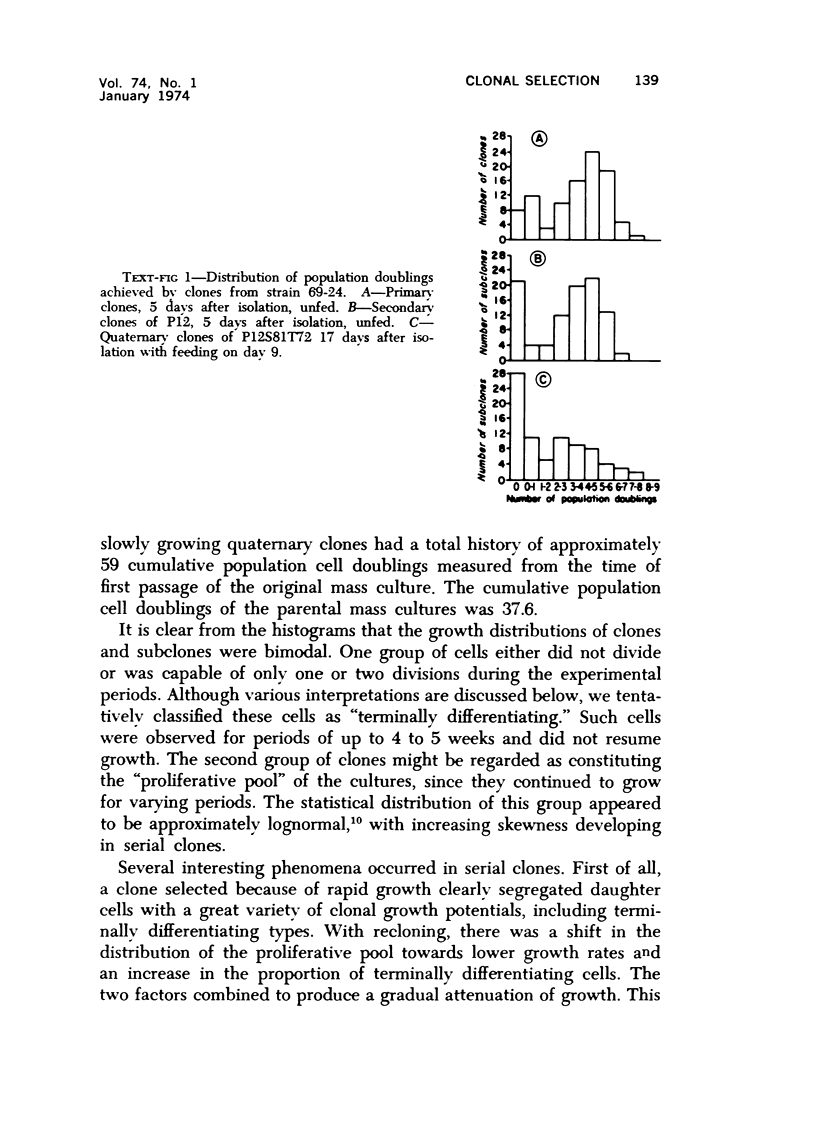
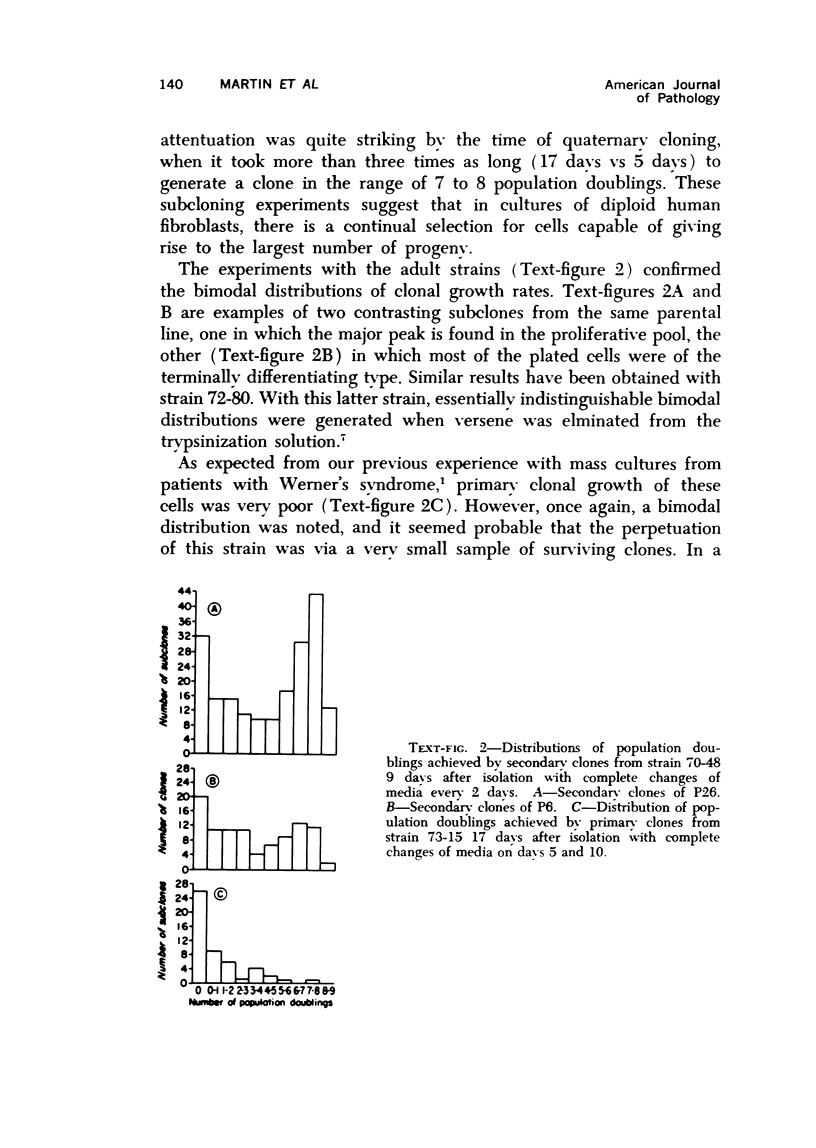
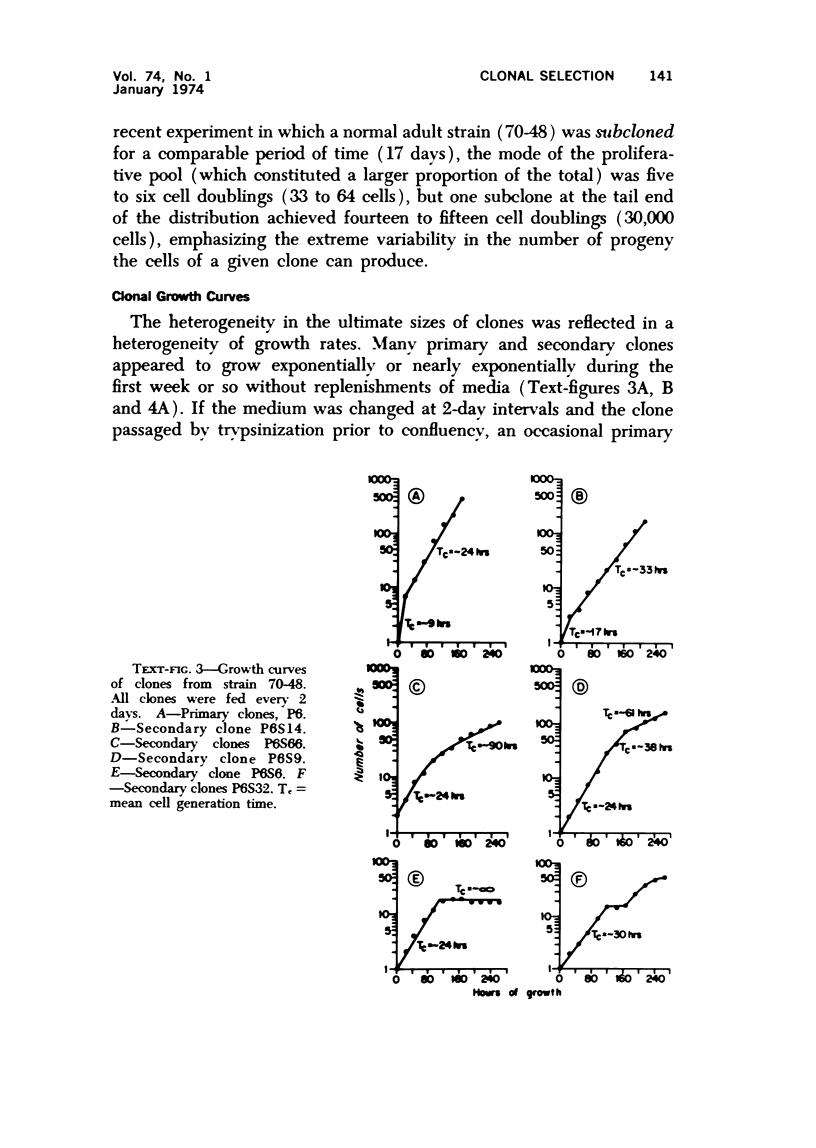
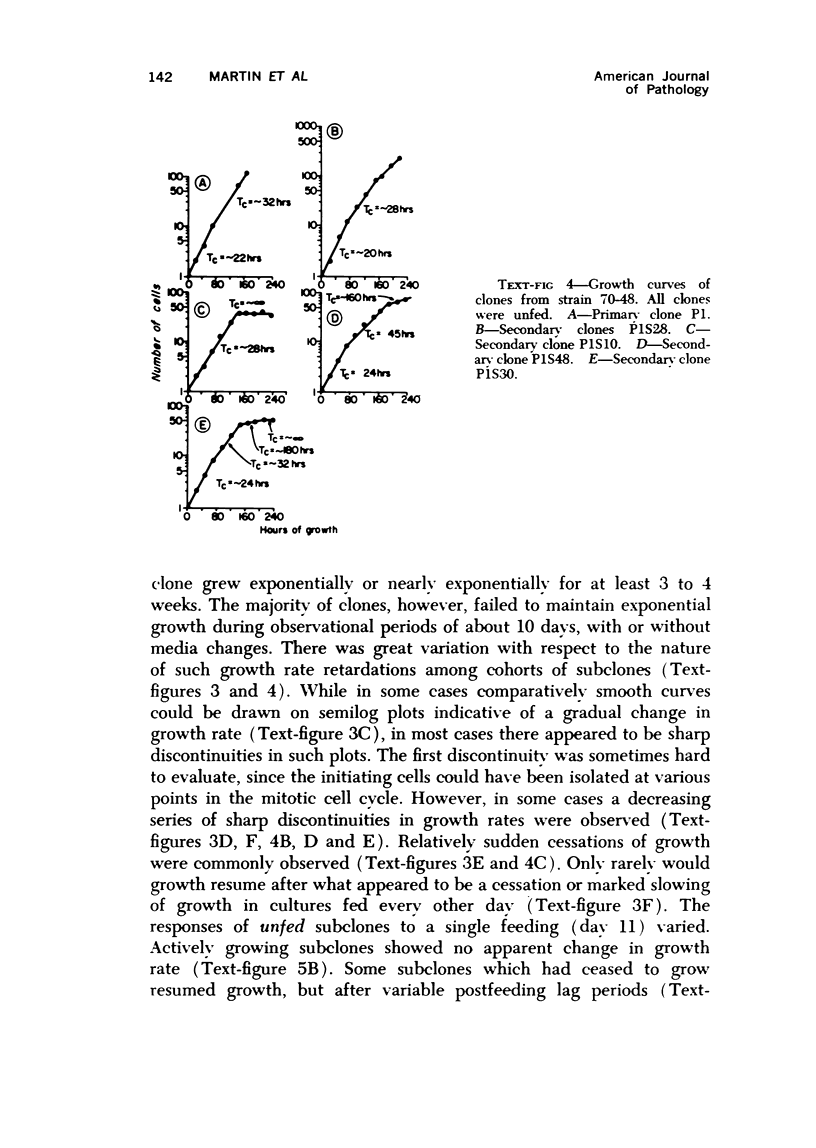
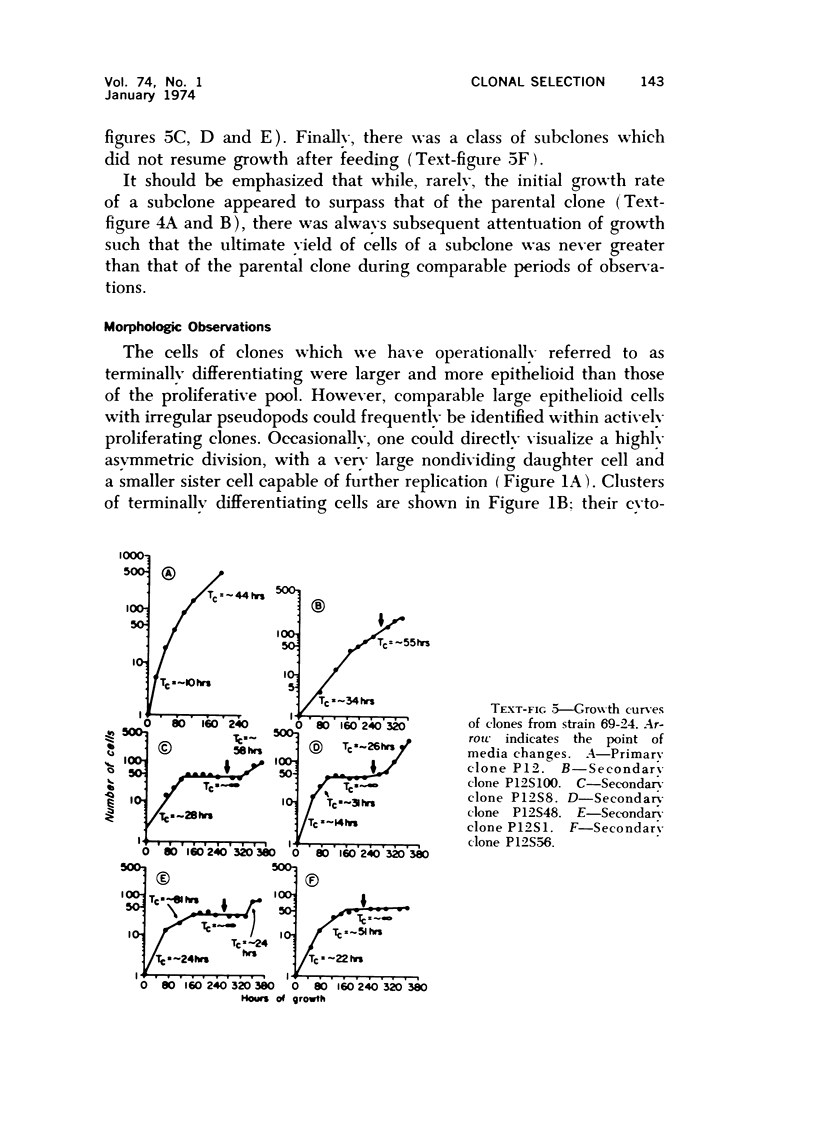
Observations of the growth kinetics and morphologies of clones and subclones of diploid human skin fibroblast cultures lead to the working hypothesis that these cells, presumably like their counterparts in healing wounds, constitute a differentiating system. There is attenuation of the growth of serial clones, with continual selection for more vigorous stem cells. The latter segregate daughter cells of varying growth potential, including a class of cells which may be regarded as terminally differentiating; we propose that such cells may be histiocytes or macrophages. These studies a) demonstrate extensive epigenetic heterogeneity in fibroblast cultures, b) suggest that hyperplastic foci may be monoclonal or oligoclonal, c) rule out a simple biologic clock mechanism as an explanation of clonal senescence, d) suggest a new approach to the analysis of various inborn errors of metabolism, such as Werner's Syndrome.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes D., Murphy D. G., Anton E. B., Barnard S. Ultrastructural and cytochemical changes in cultured human lung cells. J Ultrastruct Res. 1972 Jun;39(5):465–483. doi: 10.1016/s0022-5320(72)90114-1. [DOI] [PubMed] [Google Scholar]
- Dunn C. D. The differentiation of haemopoietic stem cells. Ser Haematol. 1971;4(4):1–71. [PubMed] [Google Scholar]
- Elsdale T., Bard J. Cellular interactions in mass cultures of human diploid fibroblasts. Nature. 1972 Mar 24;236(5343):152–155. doi: 10.1038/236152a0. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Martin G. M., Schultz A. L., Motulsky A. G. Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 1966 May;45(3):177–221. doi: 10.1097/00005792-196605000-00001. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Lagunoff D. The in vitro differentiation of mast cells. Cultures of cells from immunized mouse lymph nodes and thoracic duct lymph on fibroblast monolayers. J Cell Biol. 1967 Dec;35(3):685–697. doi: 10.1083/jcb.35.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R., Tarrant G. M. Altered enzymes in ageing human fibroblasts. Nature. 1972 Jul 7;238(5358):26–30. doi: 10.1038/238026a0. [DOI] [PubMed] [Google Scholar]
- Houck J. C., Cheng R. F. Isolation, purification, and chemical characterization of the serum mitogen for diploid human fibroblasts. J Cell Physiol. 1973 Apr;81(2):257–270. doi: 10.1002/jcp.1040810214. [DOI] [PubMed] [Google Scholar]
- Kenny G. E. Serological comparison of ten glycolytic Mycoplasma species. J Bacteriol. 1969 Jun;98(3):1044–1055. doi: 10.1128/jb.98.3.1044-1055.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny G. M. Evidence for transfer of macromolecular RNA between mammalian cells in culture. Exp Cell Res. 1971 Apr;65(2):313–324. doi: 10.1016/0014-4827(71)90007-3. [DOI] [PubMed] [Google Scholar]
- Lewis C. M., Tarrant G. M. Error theory and ageing in human diploid fibroblasts. Nature. 1972 Oct 6;239(5371):316–318. doi: 10.1038/239316a0. [DOI] [PubMed] [Google Scholar]
- Lipetz J., Cristofalo V. J. Ultrastructural changes accompanying the aging of human diploid cells in culture. J Ultrastruct Res. 1972 Apr;39(1):43–56. doi: 10.1016/s0022-5320(72)80005-4. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Cellular communication through membrane junctions. Special consideration of wound healing and cancer. Arch Intern Med. 1972 Feb;129(2):299–305. [PubMed] [Google Scholar]
- MARTIN G. M. USE OF TRIS(HYDROXYMETHYL)AMINOMETHANE BUFFERS IN CULTURES OF DIPLOID HUMAN FIBROBLASTS. Proc Soc Exp Biol Med. 1964 May;116:167–171. doi: 10.3181/00379727-116-29191. [DOI] [PubMed] [Google Scholar]
- Martin G. M. Clonal variation of derepressed phosphatase in chromosomally mosaic cell cultures from a child with Down's syndrome. Exp Cell Res. 1966 Nov-Dec;44(2):341–350. doi: 10.1016/0014-4827(66)90440-x. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A. Symposium on in vitro studies related to atherogenesis. Life histories of hyperplastoid cell lines from aorta and skin. Exp Mol Pathol. 1973 Apr;18(2):125–141. doi: 10.1016/0014-4800(73)90012-9. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Tuan A. A definitive cloning technique for human fibroblast cultures. Proc Soc Exp Biol Med. 1966 Oct;123(1):138–140. doi: 10.3181/00379727-123-31423. [DOI] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel L. E. Ageing of clones of mammalian cells. Nature. 1973 Jun 22;243(5408):441–445. doi: 10.1038/243441a0. [DOI] [PubMed] [Google Scholar]
- Orgel L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing: a correction. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1476–1476. doi: 10.1073/pnas.67.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T. G., Martin G. M. Alkaline phosphatase "constitutive" clones: evidence for de-novo heterogeneity of established human skin fibroblast strains. Exp Cell Res. 1967 Jan;45(1):72–84. doi: 10.1016/0014-4827(67)90113-9. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., De Stefano M. J. Particle recognition by cultivated macrophages. J Immunol. 1973 Mar;110(3):695–701. [PubMed] [Google Scholar]
- Robbins E., Levine E. M., Eagle H. Morphologic changes accompanying senescence of cultured human diploid cells. J Exp Med. 1970 Jun 1;131(6):1211–1222. doi: 10.1084/jem.131.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMINOVITCH L., TILL J. E., MCCULLOCH E. A. DECLINE IN COLONY-FORMING ABILITY OF MARROW CELLS SUBJECTED TO SERIAL TRANSPLANTATION INTO IRRADIATED MICE. J Cell Physiol. 1964 Aug;64:23–31. doi: 10.1002/jcp.1030640104. [DOI] [PubMed] [Google Scholar]
- WEISS P., KAVANAU J. L. A model of growth and growth control in mathematical terms. J Gen Physiol. 1957 Sep 20;41(1):1–47. doi: 10.1085/jgp.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth H. Determination and transdetermination in cells of the fruitfly. Sci Prog. 1970 Autumn;58(231):329–358. [PubMed] [Google Scholar]
- Williamson A. R., Askonas B. A. Senescence of an antibody-forming cell clone. Nature. 1972 Aug 11;238(5363):337–339. doi: 10.1038/238337a0. [DOI] [PubMed] [Google Scholar]
- Worton R. G., McCulloch E. A., Till J. E. Physical separation of hemopoietic stem cells differing in their capacity for self-renewal. J Exp Med. 1969 Jul 1;130(1):91–103. doi: 10.1084/jem.130.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]