Abstract
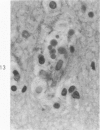
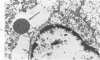
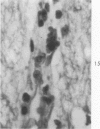
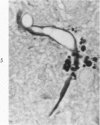
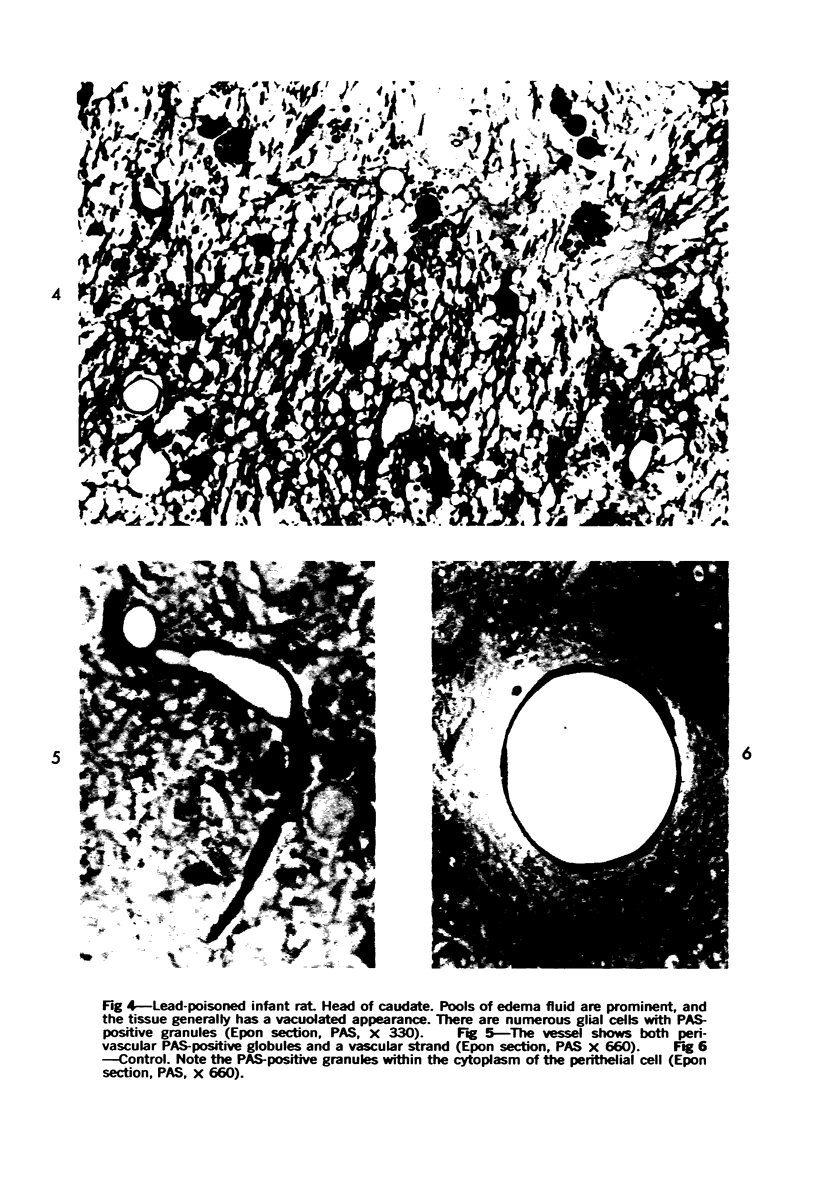
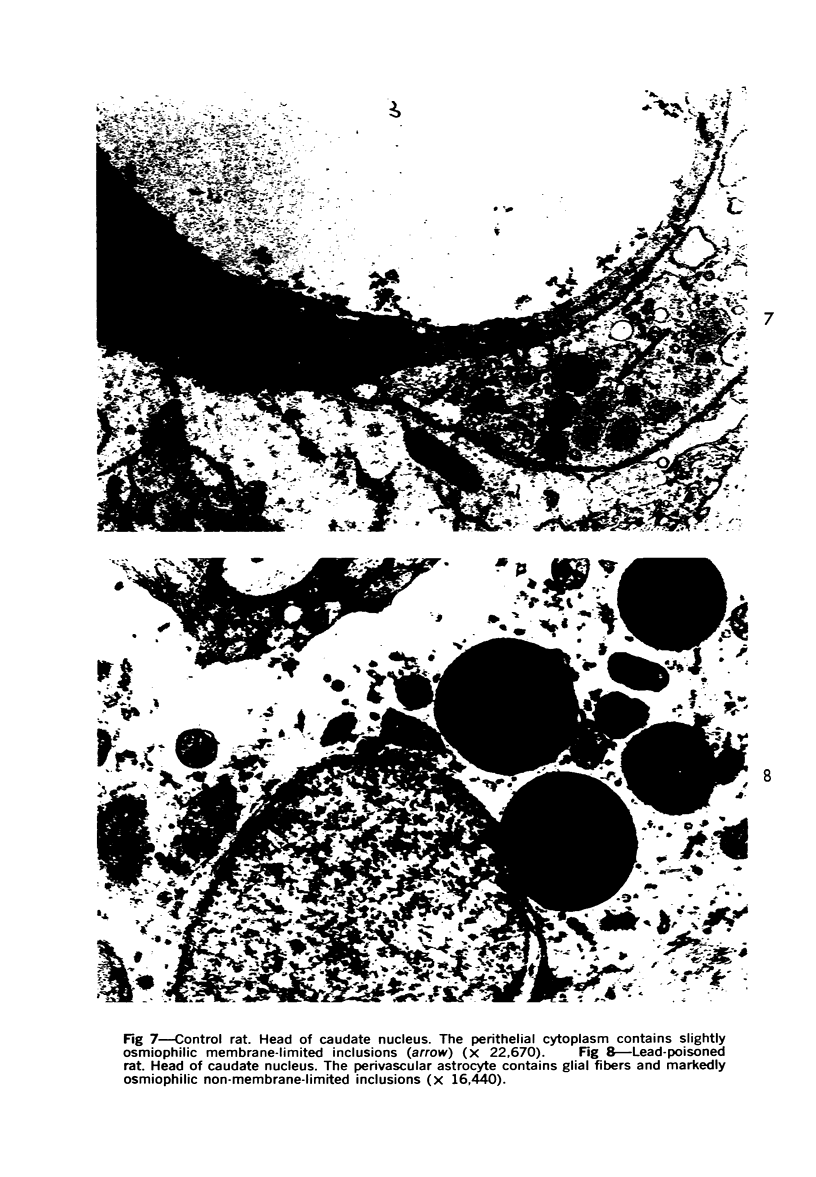
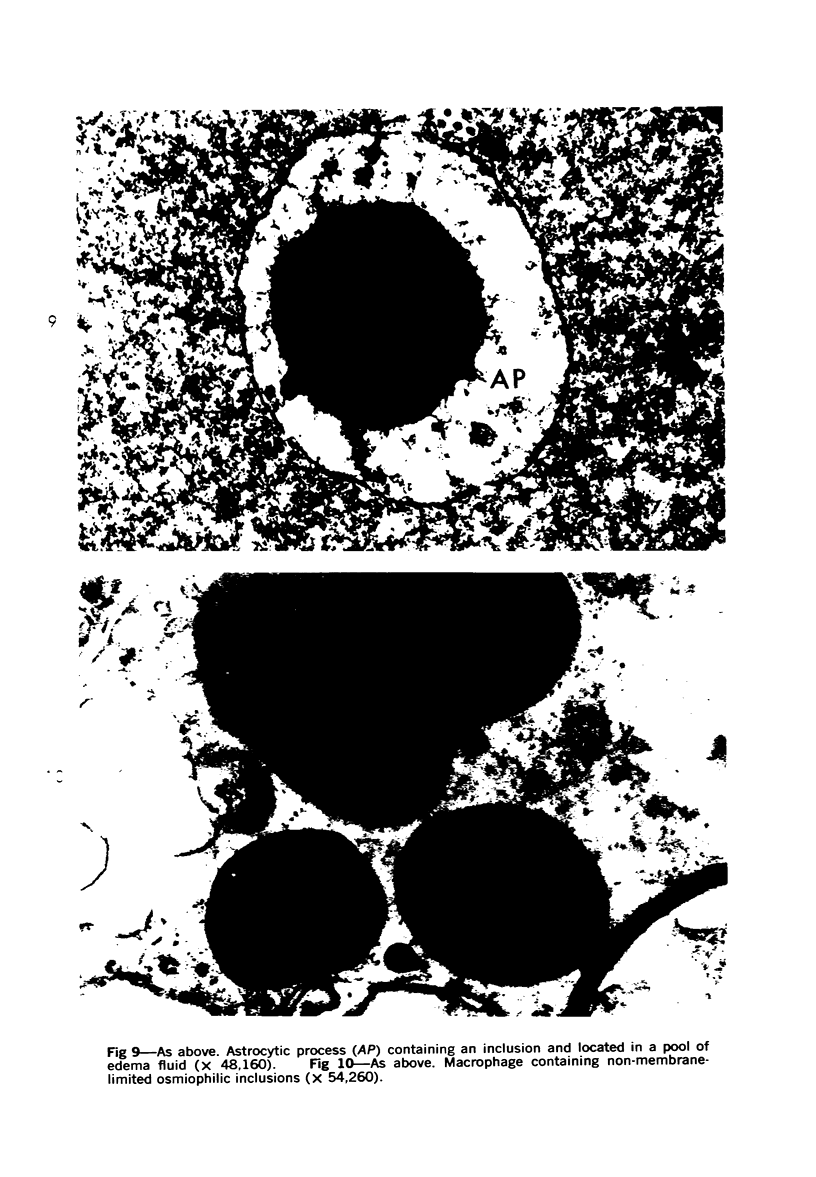
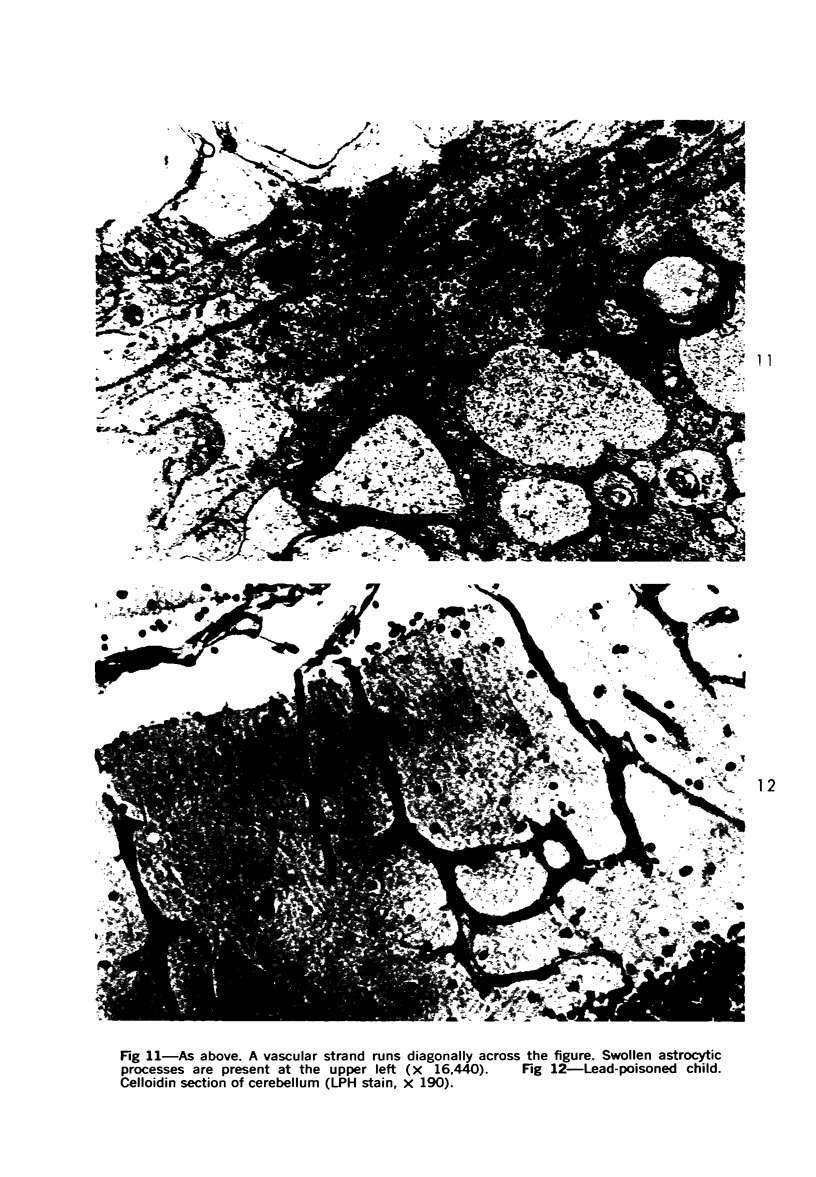
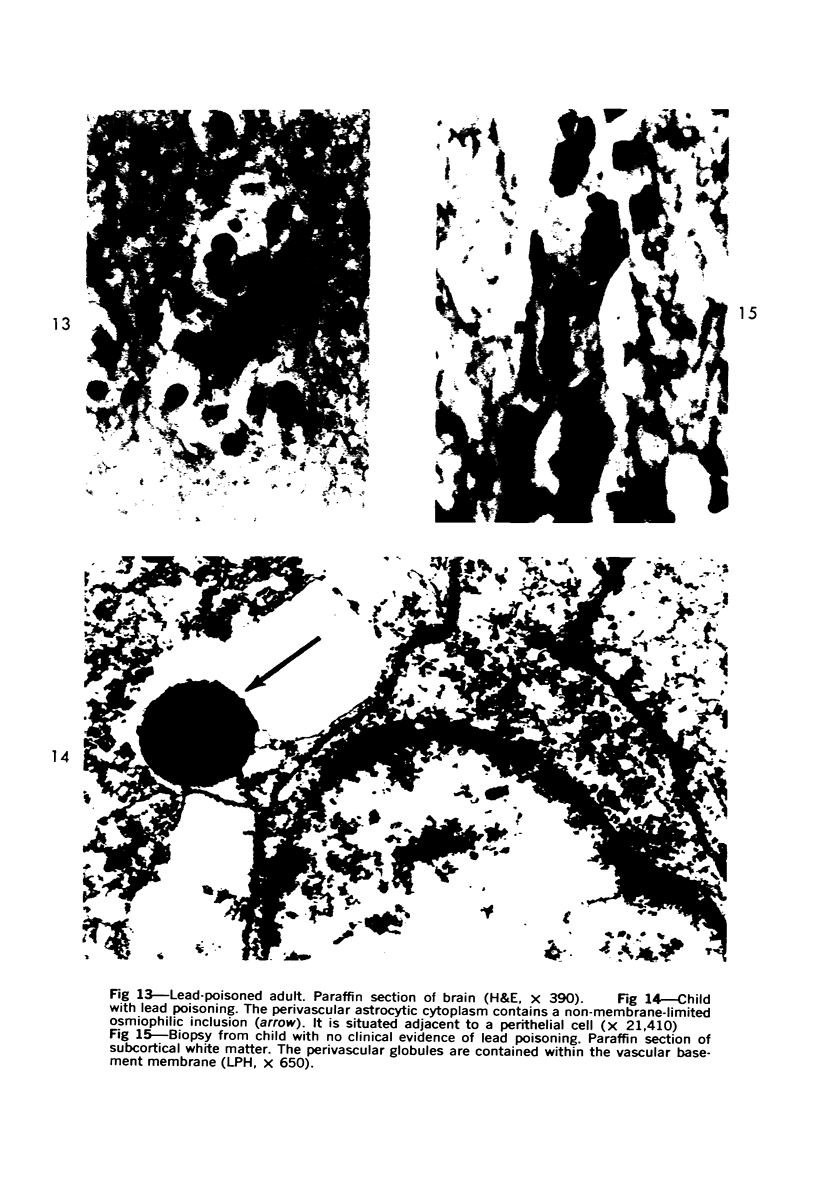
Lead encephalopathy was induced in suckling rats by administering lead to the mother. The brains were studied by light and electron microscopy, and the results were compared with observations in the human disease as well as in cases of cerebral ischemia in children. In their severe forms, both human and experimental lead encephalopathies are characterized by exudative extracellular edema and perivascular PAS-positive globules. The latter consist of osmiophilic non-membrane-limited cytoplasmic inclusions located, in the rat exclusively and in the human predominantly, in perivascular astrocytes. Intervascular strands are also found in both forms of the disease. In the rat these consist of basement membrane surrounding endothelial cytoplasm. Chemically, experimental lead encephalopathy with morphologically demonstrable edema is associated with an increase in brain water, sodium and serum albumin. Relative to the serum concentration, the increase in water is disproportionately greater than the sodium or albumin. There were no demonstrable changes in chloride or potassium.
Full text
PDF






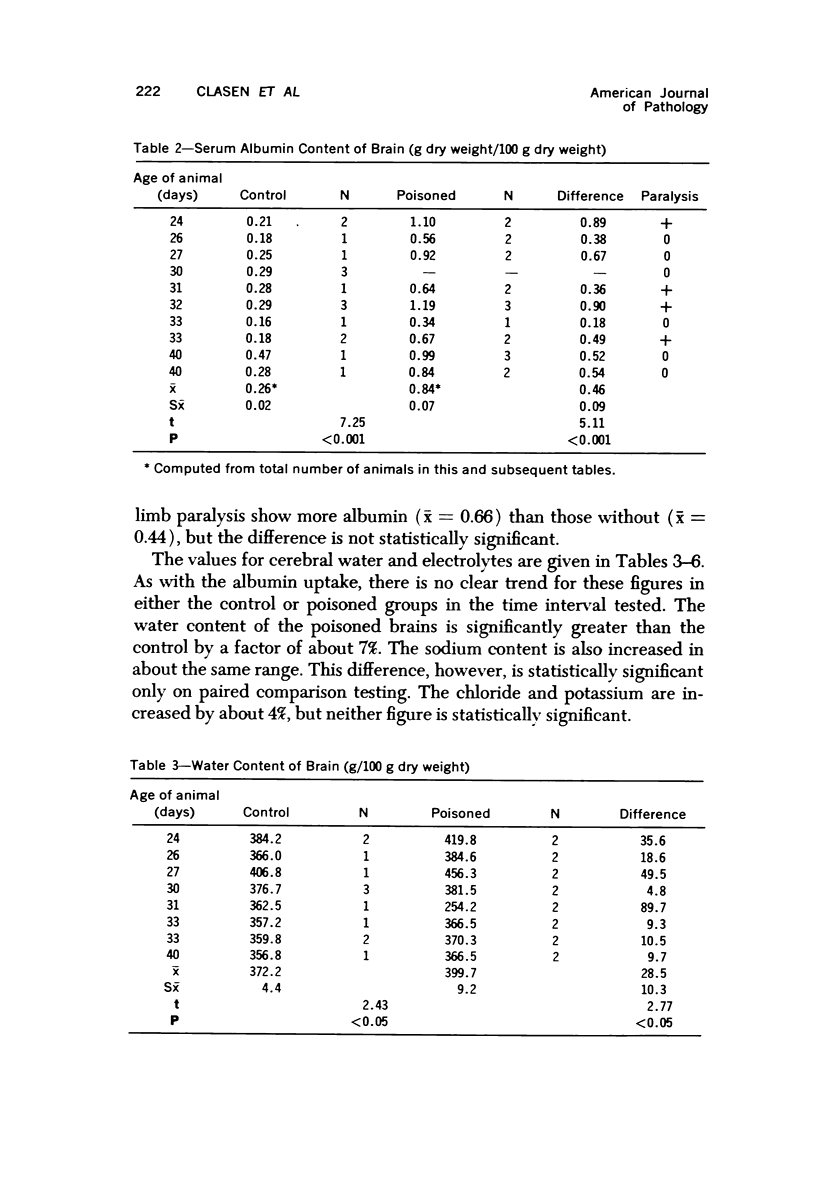
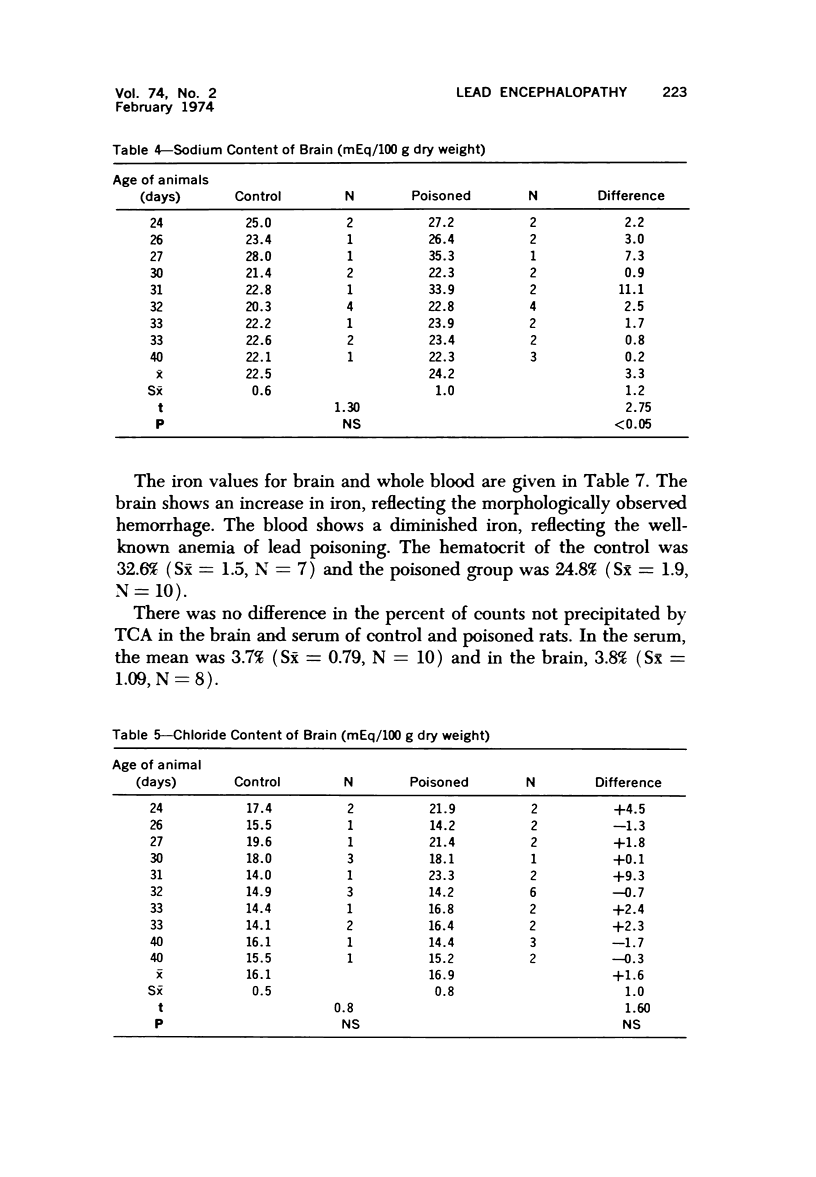
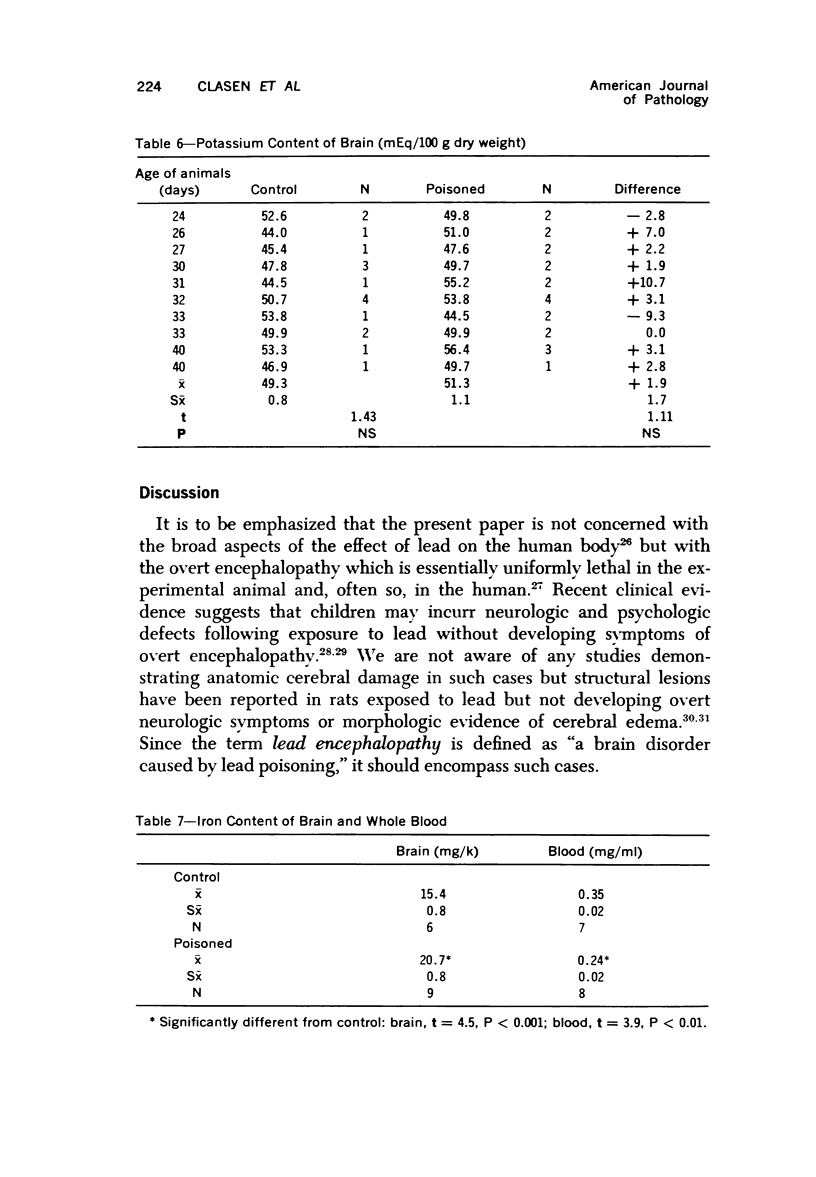










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battifora H., Eisenstein R., Laing G. H., McCreary P. The kidney in experimental magnesium deprivation. a morphologic and biochemical study. Am J Pathol. 1966 Mar;48(3):421–437. [PMC free article] [PubMed] [Google Scholar]
- Broman T., Gröntoft O., Steinwall O. Comparative intravital and postmortem studies on chemically induced blood-brain barrier damage tested with trypan blue. Acta Neurol Scand. 1965;41(5):527–538. doi: 10.1111/j.1600-0404.1965.tb04742.x. [DOI] [PubMed] [Google Scholar]
- CAMMERMEYER J. A comparative study of intervascular connective tissue strands in the central nervous system. J Comp Neurol. 1960 Apr;114:189–208. doi: 10.1002/cne.901140206. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970 Jan;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- Chang L. W., Hartmann H. A. Electron microscopic histochemical study on the localization and distribution of mercury in the nervous system after mercury intoxication. Exp Neurol. 1972 Apr;35(1):122–137. doi: 10.1016/0014-4886(72)90064-7. [DOI] [PubMed] [Google Scholar]
- Clasen R. A., Pandolfi S., Hass G. M. Vital staining, serum albumin and the blood-brain barrier. J Neuropathol Exp Neurol. 1970 Apr;29(2):266–284. [PubMed] [Google Scholar]
- Clasen R. A., Pandolfi S., Russell J., Stuart D., Hass G. M. Hypothermia and hypotension in experimental cerebral edema. Arch Neurol. 1968 Nov;19(5):472–486. doi: 10.1001/archneur.1968.00480050042004. [DOI] [PubMed] [Google Scholar]
- Coffin R., Phillips J. L., Staples W. I., Spector S. Treatment of lead encephalopathy in children. J Pediatr. 1966 Aug;69(2):198–206. doi: 10.1016/s0022-3476(66)80320-7. [DOI] [PubMed] [Google Scholar]
- Cohen N., Kneip T. J., Goldstein D. H., Muchmore E. A. The juvenile baboon as a model for studies of lead poisoning in children. J Med Primatol. 1972;1(3):142–155. doi: 10.1159/000460377. [DOI] [PubMed] [Google Scholar]
- De la Burdé B., Choate M. S., Jr Does asymptomatic lead exposure in children have latent sequelae? J Pediatr. 1972 Dec;81(6):1088–1091. doi: 10.1016/s0022-3476(72)80236-1. [DOI] [PubMed] [Google Scholar]
- Eisenberg H. M., Barlow C. F., Lorenzo A. V. Effect of dexamethasone on altered brain vascular permeability. Arch Neurol. 1970 Jul;23(1):18–22. doi: 10.1001/archneur.1970.00480250022004. [DOI] [PubMed] [Google Scholar]
- Goyer R. A., Rhyne B. C. Pathological effects of lead. Int Rev Exp Pathol. 1973;12:1–77. [PubMed] [Google Scholar]
- HASS G. M., BROWN D. V., EISENSTEIN R., HEMMENS A. RELATIONS BETWEEN LEAD POISONING IN RABBIT AND MAN. Am J Pathol. 1964 Nov;45:691–727. [PMC free article] [PubMed] [Google Scholar]
- Hartmann J. F. High sodium content of cortical astrocytes. Arch Neurol. 1966 Dec;15(6):633–642. doi: 10.1001/archneur.1966.00470180073008. [DOI] [PubMed] [Google Scholar]
- Hindo W. A., Clasen R. A., Rayudu G. V. Technetium in cryogenic cerebral injury and edema. Arch Neurol. 1972 Dec;27(6):526–534. doi: 10.1001/archneur.1972.00490180062014. [DOI] [PubMed] [Google Scholar]
- Hopkins A. Experimental lead poisoning in the baboon. Br J Ind Med. 1970 Apr;27(2):130–140. doi: 10.1136/oem.27.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZMAN R. Electrolyte distribution in mammalian central nervous system. Are glia high sodium cells? Neurology. 1961 Jan;11:27–36. doi: 10.1212/wnl.11.1.27. [DOI] [PubMed] [Google Scholar]
- Olsson Y., Hossmann K. A. Fine structural localization of exudated protein tracers in the brain. Acta Neuropathol. 1970;16(2):103–116. doi: 10.1007/BF00687665. [DOI] [PubMed] [Google Scholar]
- Oyasu R., Battifora H. A., Clasen R. A., McDonald J. H., Hass G. M. Induction of cerebral gliomas in rats with dietary lead subacetate and 2-acetylaminofluorene. Cancer Res. 1970 May;30(5):1248–1261. [PubMed] [Google Scholar]
- Pentschew A., Garro F. Lead encephalo-myelopathy of the suckling rat and its implications on the porphyrinopathic nervous diseases. With special reference to the permeability disorders of the nervous system's capillaries. Acta Neuropathol. 1966 Jun 1;6(3):266–278. doi: 10.1007/BF00687857. [DOI] [PubMed] [Google Scholar]
- Pentschew A. Morphology and morphogenesis of lead encephalopathy. Acta Neuropathol. 1965 Nov 18;5(2):133–160. doi: 10.1007/BF00686515. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I., Johnson M. G. Neuropathologic changes produced in suckling mice by adding lead to the maternal diet. Arch Pathol. 1968 Jun;85(6):640–648. [PubMed] [Google Scholar]
- Sauer R. M., Zook B. C., Garner F. M. Demyelinating encephalomyelopathy associated with lead poisoning in nonhuman primates. Science. 1970 Sep 11;169(3950):1091–1093. doi: 10.1126/science.169.3950.1091. [DOI] [PubMed] [Google Scholar]
- Steinwall O., Klatzo I. Selective vulnerability of the blood-brain barrier in chemically induced lesions. J Neuropathol Exp Neurol. 1966 Oct;25(4):542–559. doi: 10.1097/00005072-196610000-00004. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Dallenbach F. D., Thomas M. Considerations on the development of experimental lead encephalopathy. Virchows Arch A Pathol Pathol Anat. 1971;352(1):61–74. doi: 10.1007/BF00549764. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Dallenbach F. D., Thomas M. The distribution of radioactive lead (210Pb) in the cerebellum of developing rats. J Pathol. 1973 Jan;109(1):45–50. doi: 10.1002/path.1711090106. [DOI] [PubMed] [Google Scholar]
- Zook B. C., Sauer R. M., Bush M., Gray C. W. Lead poisoning in zoo-dwelling primates. Am J Phys Anthropol. 1973 Mar;38(2):415–423. doi: 10.1002/ajpa.1330380242. [DOI] [PubMed] [Google Scholar]
- Zook B. C., Sauer R. M., Garner F. M. Acute amaurotic epilepsy caused by lead poisoning in nonhuman primates. J Am Vet Med Assoc. 1972 Sep 15;161(6):683–686. [PubMed] [Google Scholar]