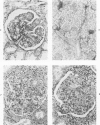
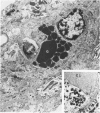
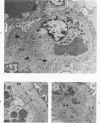
Abstract
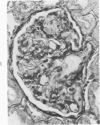
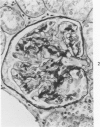
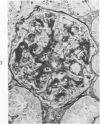
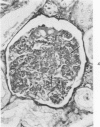
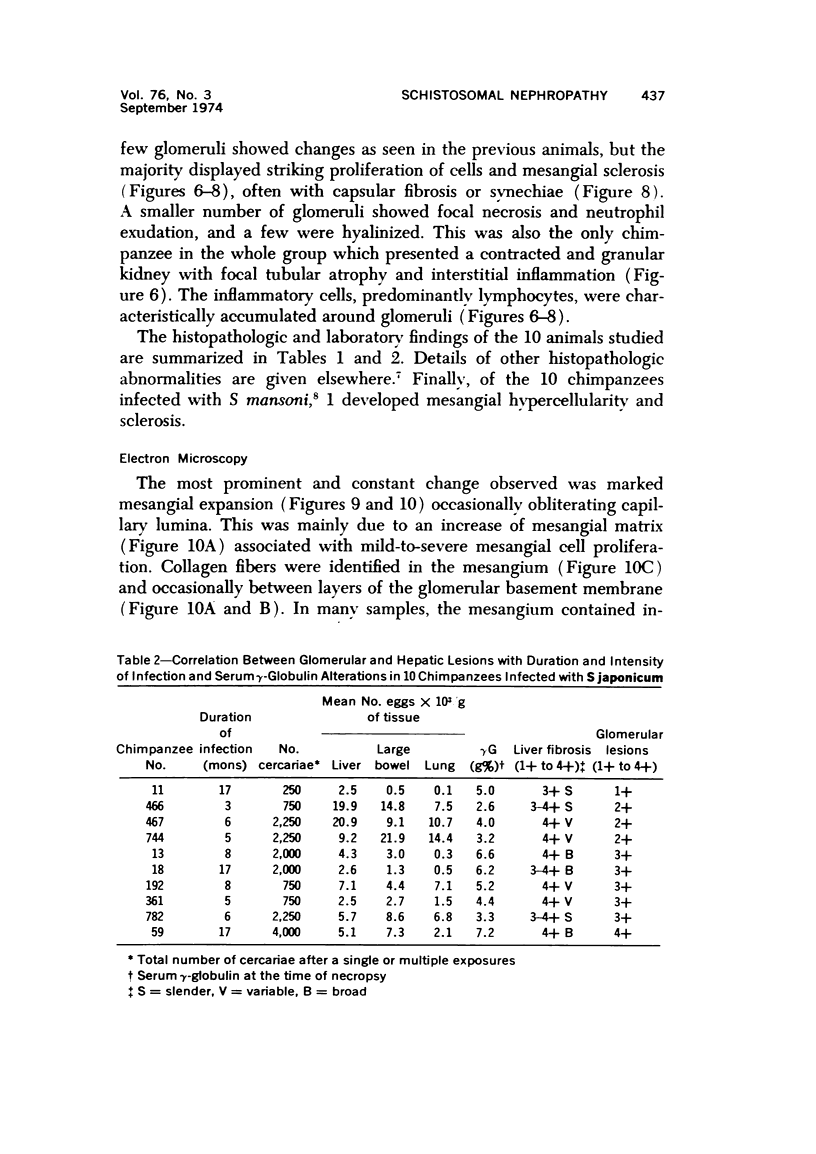
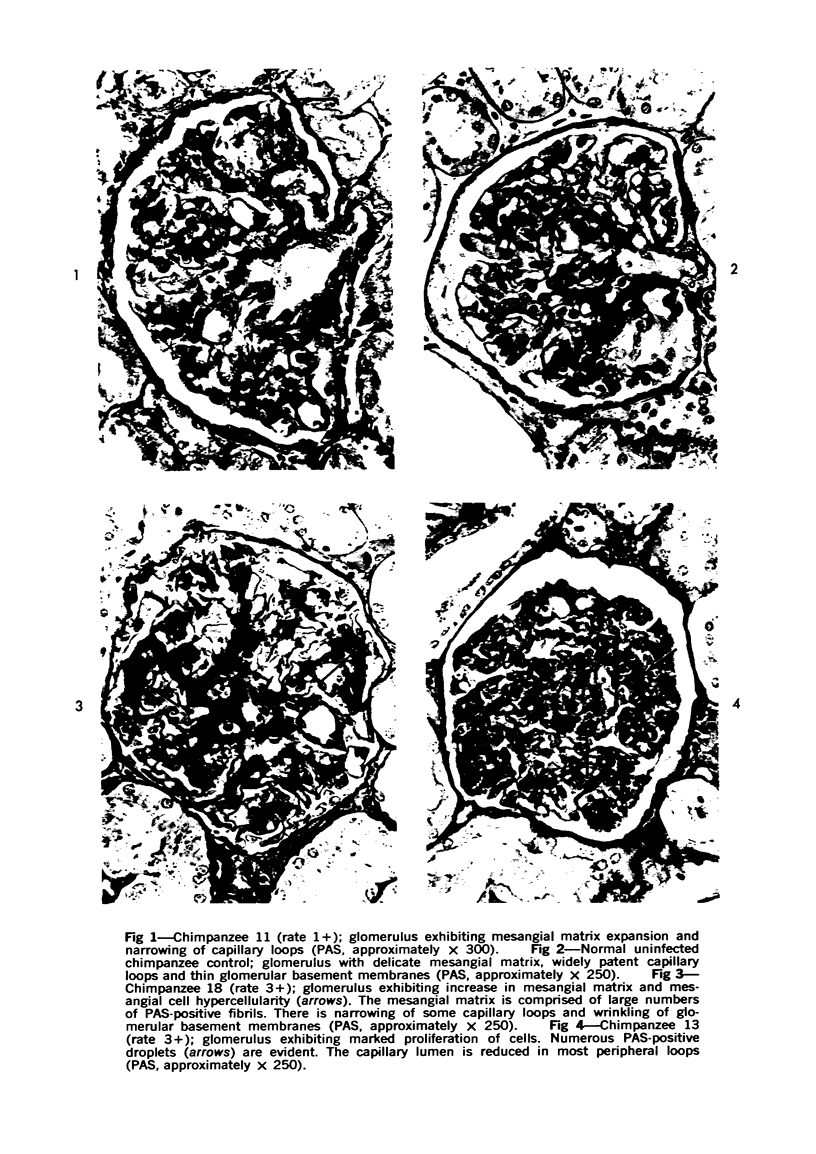
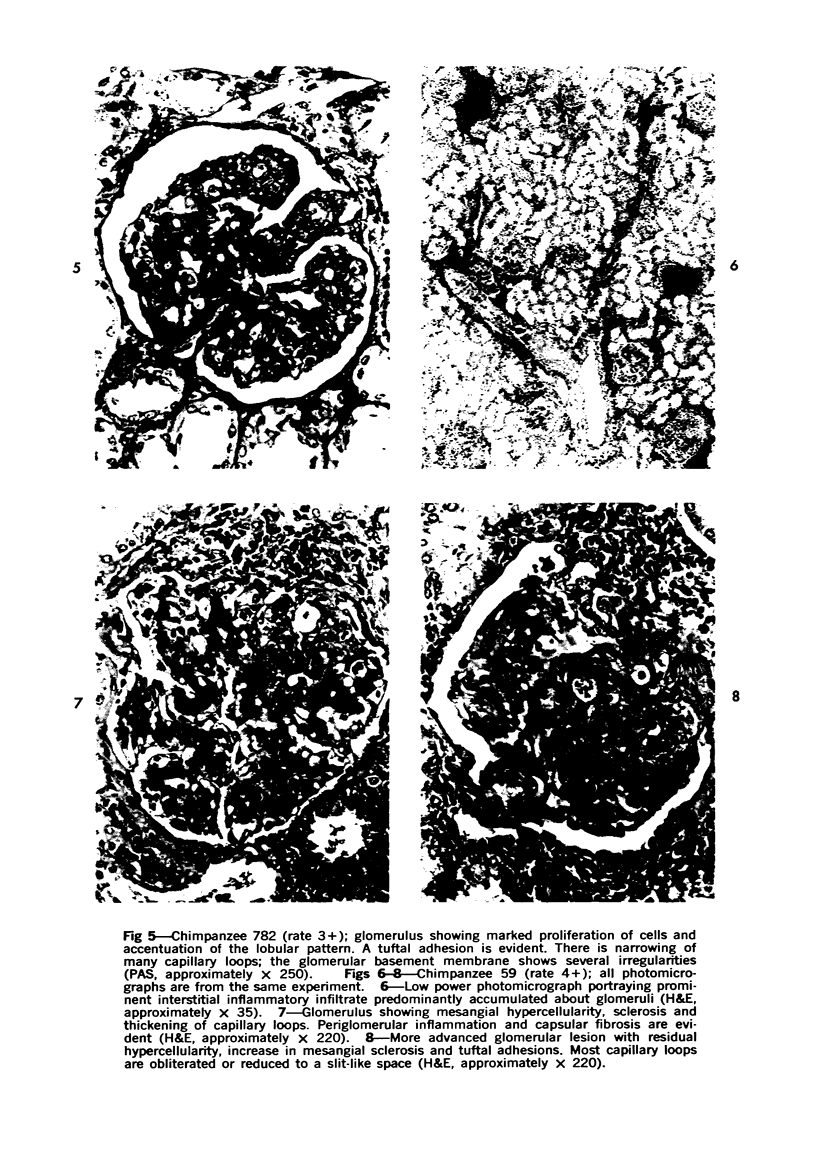
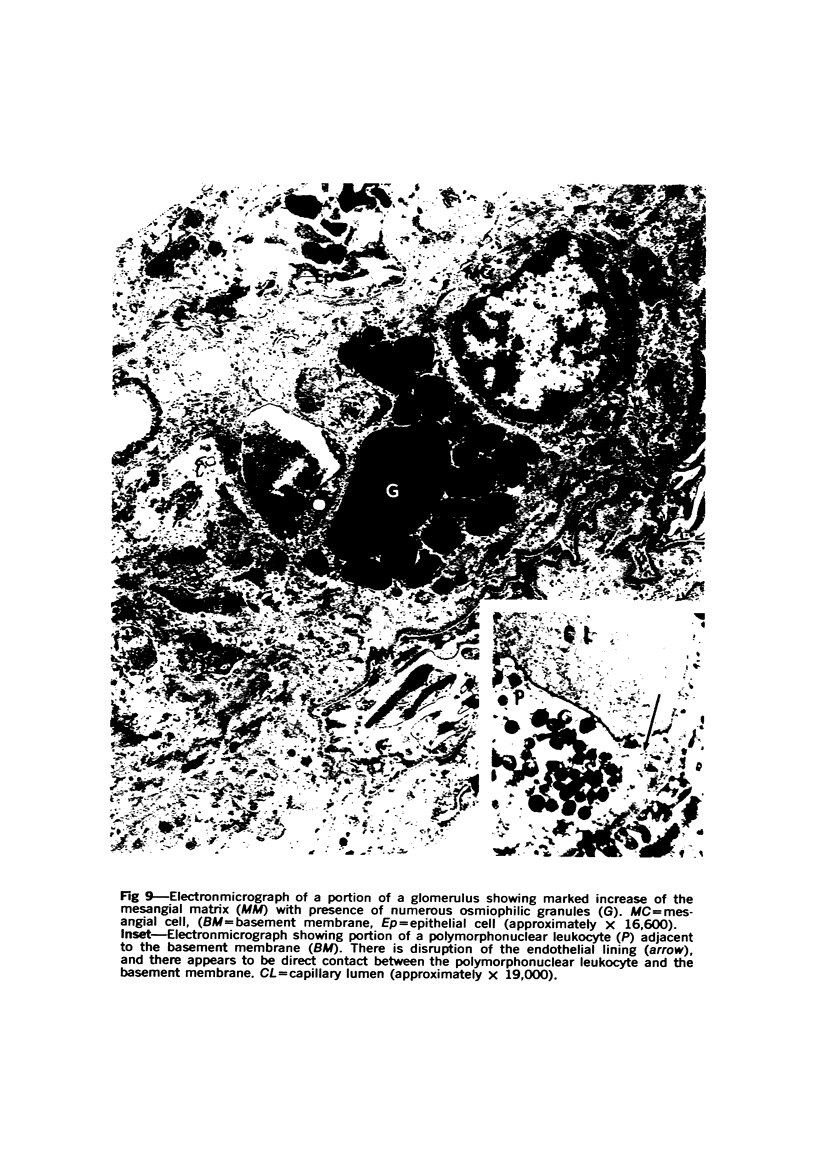
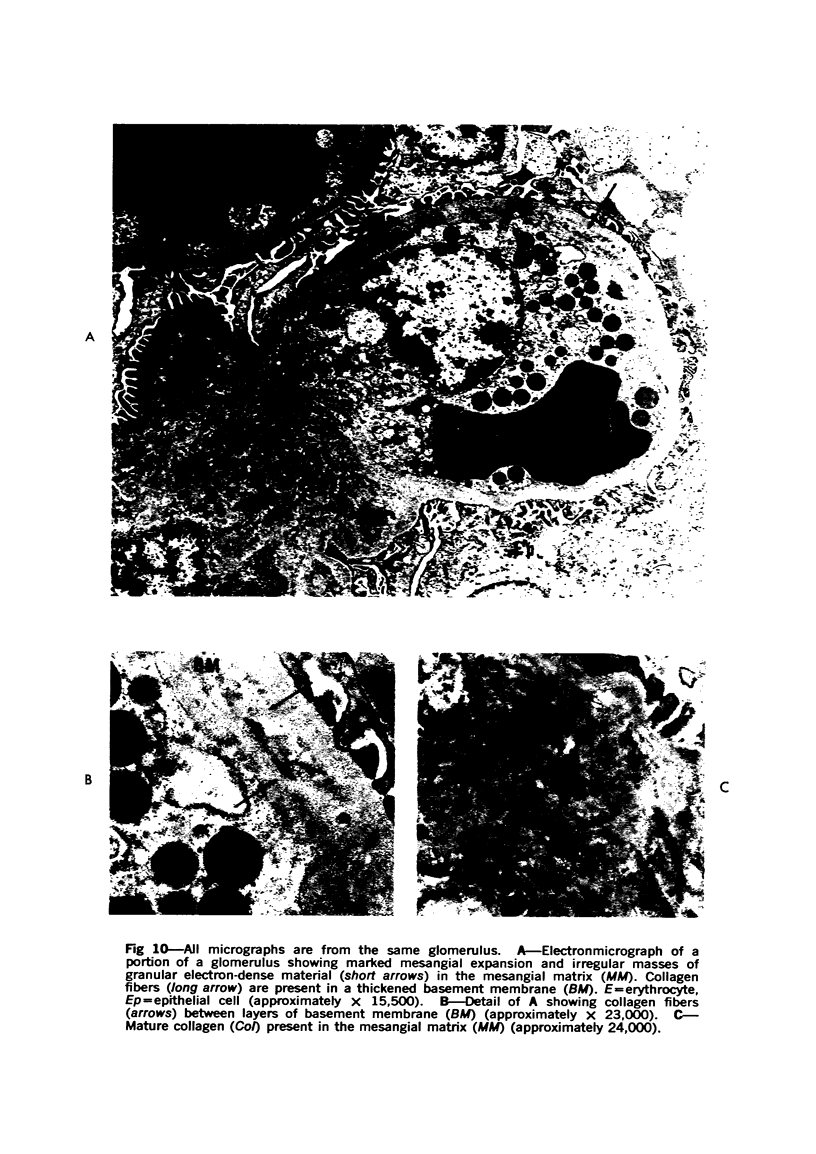
The glomerular lesions induced in 10 chimpanzees infected with variable numbers of Schistosoma japonicum cercariae were studied by means of light and electron microscopy and fluorescent antibody technic. Ten animals served as controls; 5 were uninfected and 5 were only lightly infected. The animals were observed for periods ranging from 3 to 17 months, and by the time of sacrifice, all had developed advanced liver fibrosis. In general, the degree of glomerular injury was related to infection intensity and degree and duration of portal liver fibrosis. Some animals had terminal BUN elevation and slight proteinuria. By light and electron microscopy, in the initial stages, only part of the glomeruli were involved and exhibited mesangial matrix expansion and mesangial cell proliferation with intracellular hyaline droplets. At later stages, a larger number of glomeruli were affected and exhibited diffuse hypercellularity, glomerular basement thickening, mesangial sclerosis and less often, focal necrosis, crescent formation, synechiae and global hyalinization. In addition, there were discrete electron-dense deposits localized in the mesangial area in some glomeruli. Immunofluorescent studies utilizing antisera to chimpanzee γ-globulin and complement (C3) and to human properdin disclosed only faint deposits of C3, apparently in mesangial areas. The association of hepatosplenic schistosomiasis and nephropathy, the possible role of schistosomal antigen and the mechanism(s) of such glomerular injuries are reviewed and compared with the disease in humans and other host species infected with Schistosoma.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade Z. A., Andrade S. G., Sadigursky M. Renal changes in patients with hepatosplenic schistosomiasis. Am J Trop Med Hyg. 1971 Jan;20(1):77–83. doi: 10.4269/ajtmh.1971.20.77. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., Susin M. Renal changes in mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1974 May;23(3):400–403. doi: 10.4269/ajtmh.1974.23.400. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., de Queiroz A. C. Lesões renais na esquistossomose hepatesplênica. Rev Inst Med Trop Sao Paulo. 1968 Jan-Feb;10(1):36–40. [PubMed] [Google Scholar]
- Berggren W. L., Weller T. H. Immunoelectrophoretic demonstration of specific circulating antigen in animals infected with Schistosoma mansoni. Am J Trop Med Hyg. 1967 Sep;16(5):606–612. doi: 10.4269/ajtmh.1967.16.606. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COONS A. H., KAPLAN M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1–13. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W. Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull World Health Organ. 1968;39(2):328–331. [PMC free article] [PubMed] [Google Scholar]
- Fisher E. R., Perez-Stable E. Cirrhotic (hepatic) lobular glomerulonephritis. Correlation of ultrastructural and clinical features. Am J Pathol. 1968 Apr;52(4):869–889. [PMC free article] [PubMed] [Google Scholar]
- Gold R., Rosen F. S., Weller T. H. A specific circulating antigen in hamsters infected with Schistosoma mansoni. Detection of antigen in serum and urine, and correlation between antigenic concentration and worm burden. Am J Trop Med Hyg. 1969 Jul;18(4):545–552. doi: 10.4269/ajtmh.1969.18.545. [DOI] [PubMed] [Google Scholar]
- Hillyer G. V. Deoxyribonucleic acid (DNA) and antibodies to DNA in the serum of hamsters and man infected with schistosomes. Proc Soc Exp Biol Med. 1971 Mar;136(3):880–883. doi: 10.3181/00379727-136-35386. [DOI] [PubMed] [Google Scholar]
- Hillyer G. V., Lewert R. M. Studies on renal pathology in hamsters infected with Schistosoma mansoni and S. japonicum. Am J Trop Med Hyg. 1974 May;23(3):404–411. doi: 10.4269/ajtmh.1974.23.404. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle R. B., Ward P. A., Lindsley H. B., Sadun E. H., Johnson A. J., Berkaw R. E., Hildebrandt P. K. Experimental infections with African Trypanosomes. VI. Glomerulonephritis involving the alternate pathway of complement activation. Am J Trop Med Hyg. 1974 Jan;23(1):15–26. [PubMed] [Google Scholar]
- Queiroz P. F., Brito E., Martinelli R., Rocha H. Nephrotic syndrome in patients with Schistosoma mansoni infection. Am J Trop Med Hyg. 1973 Sep;22(5):622–628. doi: 10.4269/ajtmh.1973.22.622. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadun E. H., Von Lichtenberg F., Cheever A. W., Erickson D. G., Hickman R. L. Experimental infection with Schistosoma haematobium in chimpanzees. Am J Trop Med Hyg. 1970 May;19(3):427–458. doi: 10.4269/ajtmh.1970.19.427. [DOI] [PubMed] [Google Scholar]
- Sadun E. H., von Lichtenberg F., Cheever A. W., Erickson D. G. Schistosomiasis mansoni in the chimpanzee. The natural history of chronic infections after single and multiple exposures. Am J Trop Med Hyg. 1970 Mar;19(2):258–277. [PubMed] [Google Scholar]
- Schmidt R. E., Butler T. M. Glomerulonephritis and uremia in a chimpanzee. J Med Primatol. 1973;2(3):144–154. doi: 10.1159/000460318. [DOI] [PubMed] [Google Scholar]
- Von Lichtenberg F., Sadun E. H., Cheever A. W., Erickson D. G., Johnson A. J., Boyce H. W. Experimental infection with Schistosoma japonicum in chimpanzees. Am J Trop Med Hyg. 1971 Nov;20(6):850–893. doi: 10.4269/ajtmh.1971.20.850. [DOI] [PubMed] [Google Scholar]
- Ward P. A. Conran PB: Immunopathologic studies of simian malaria. Mil Med. 1966 Sep;131(9 Suppl):1225–1232. [PubMed] [Google Scholar]
- Warren K. S. The immunopathogenesis of schistosomiasis: a multidisciplinary approach. Trans R Soc Trop Med Hyg. 1972;66(3):417–434. doi: 10.1016/0035-9203(72)90273-8. [DOI] [PubMed] [Google Scholar]
- da Silva L. C., de Brito T., Camargo M. E., de Boni D. R., Lopes J. D., Gunji J. Kidney biopsy in the hepatosplenic form of infection with Schistosoma mansoni in man. Bull World Health Organ. 1970;42(6):907–910. [PMC free article] [PubMed] [Google Scholar]