Abstract
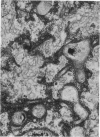
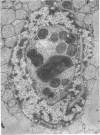
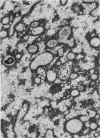
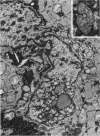
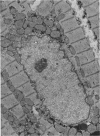
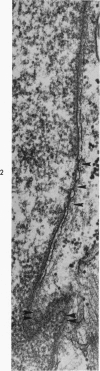
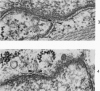
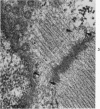
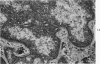
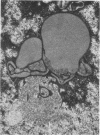
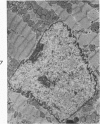
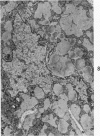
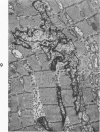
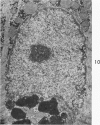
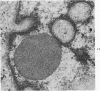
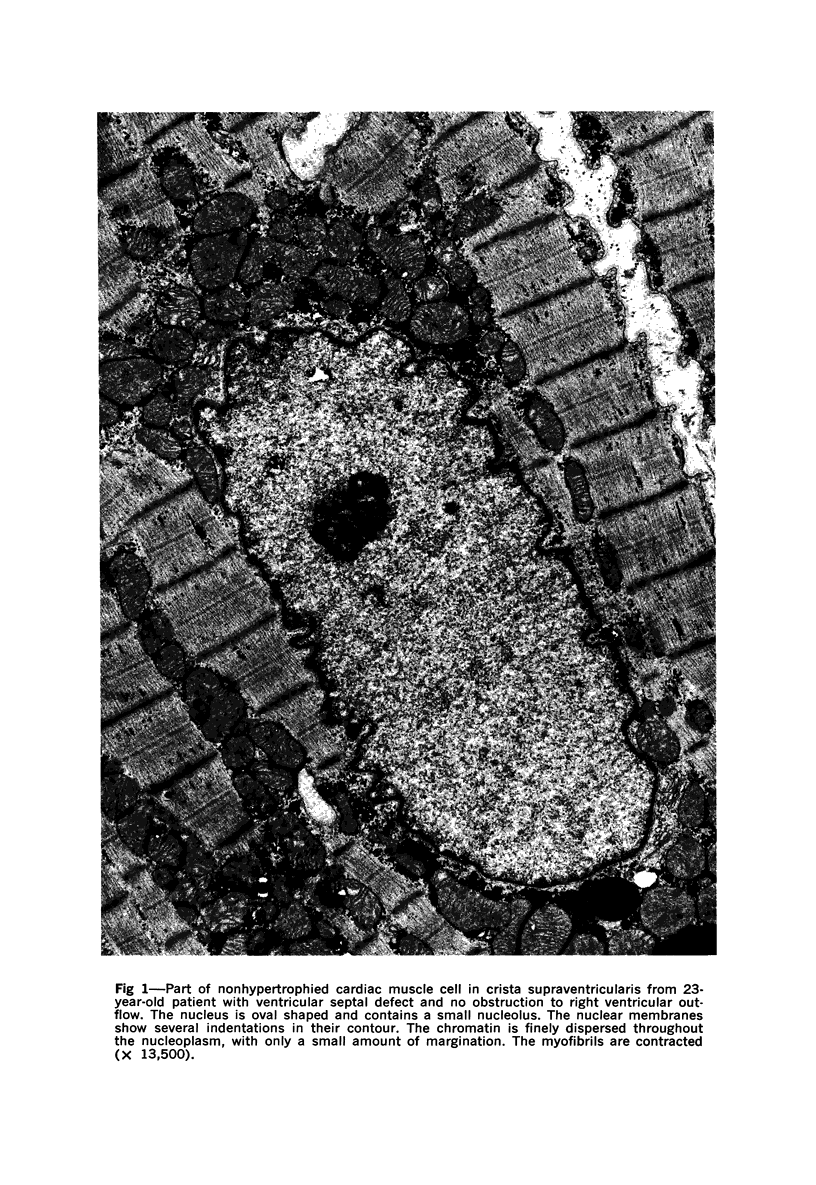
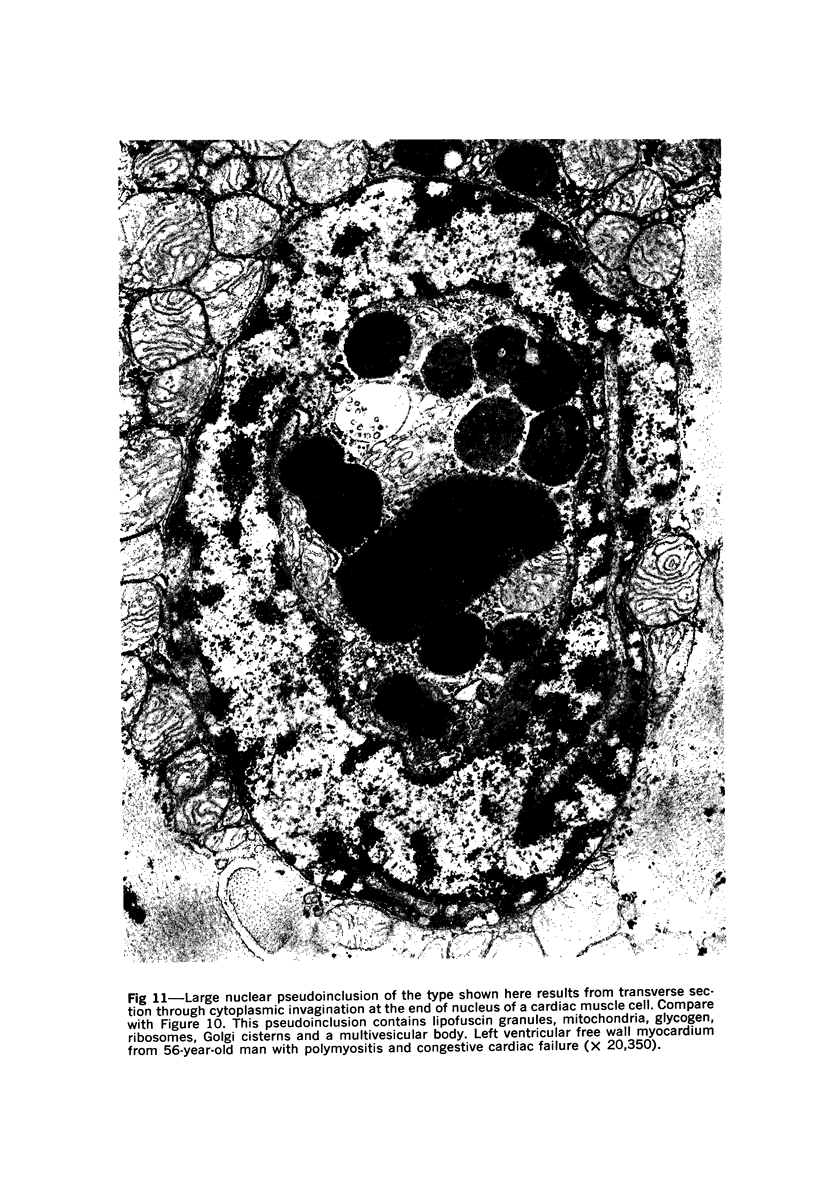
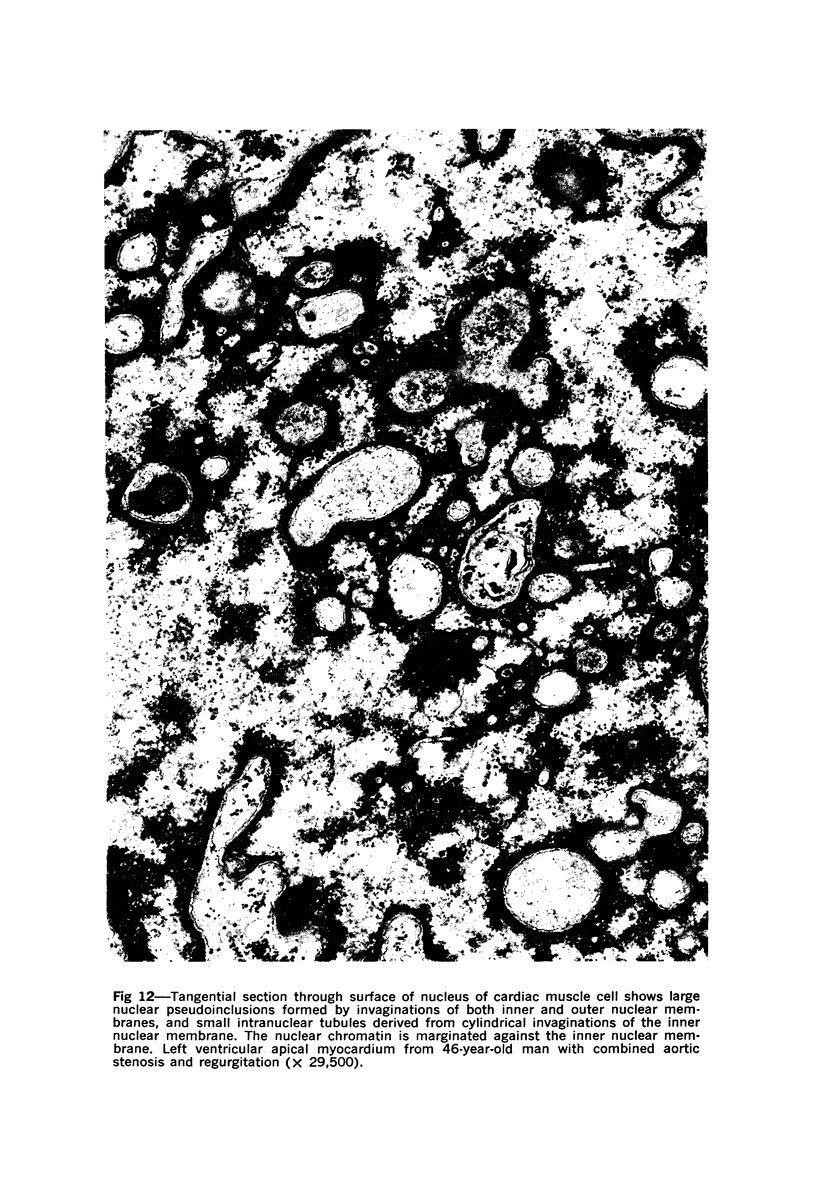
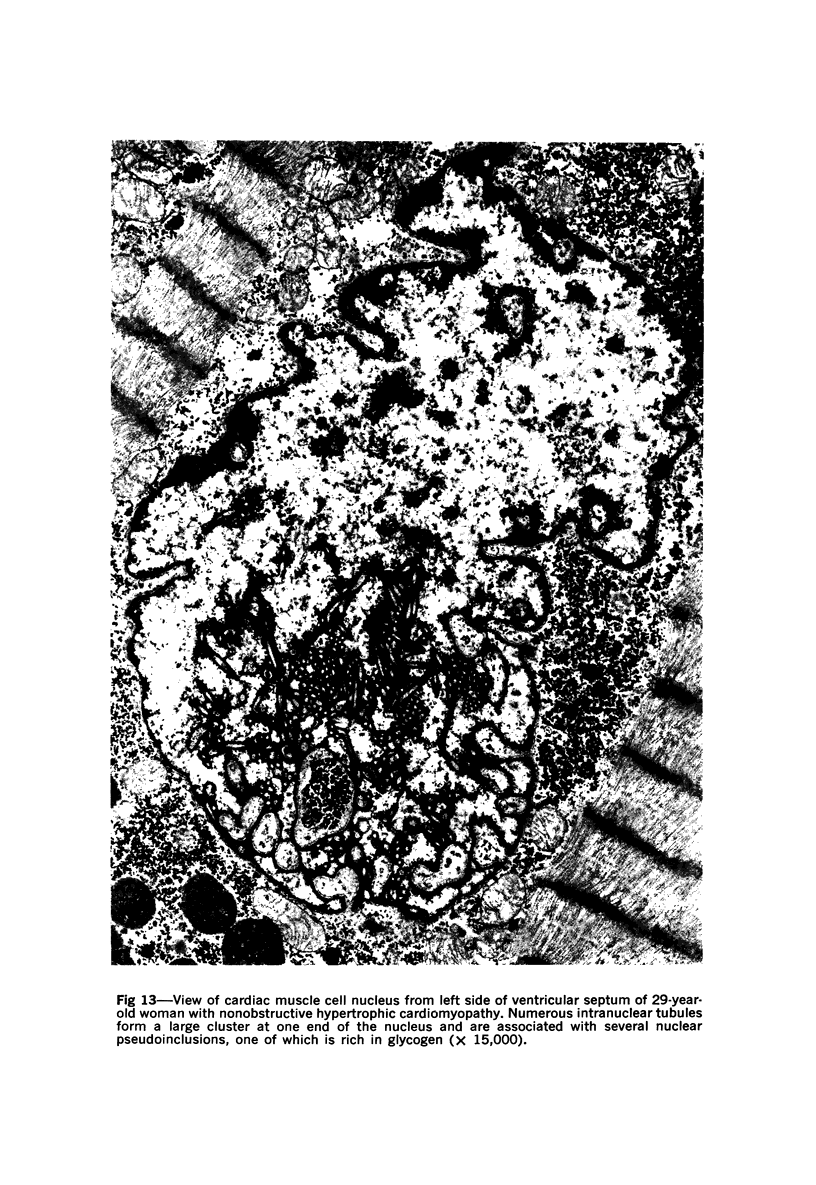
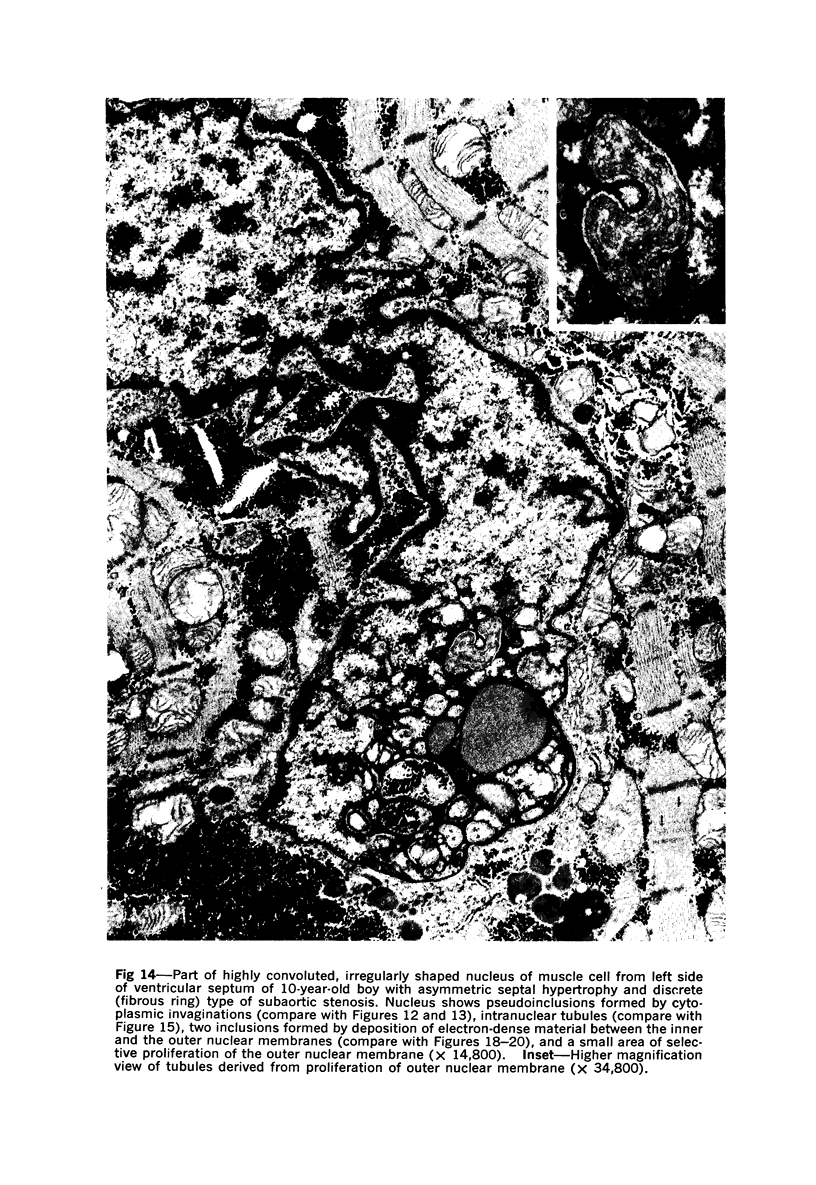
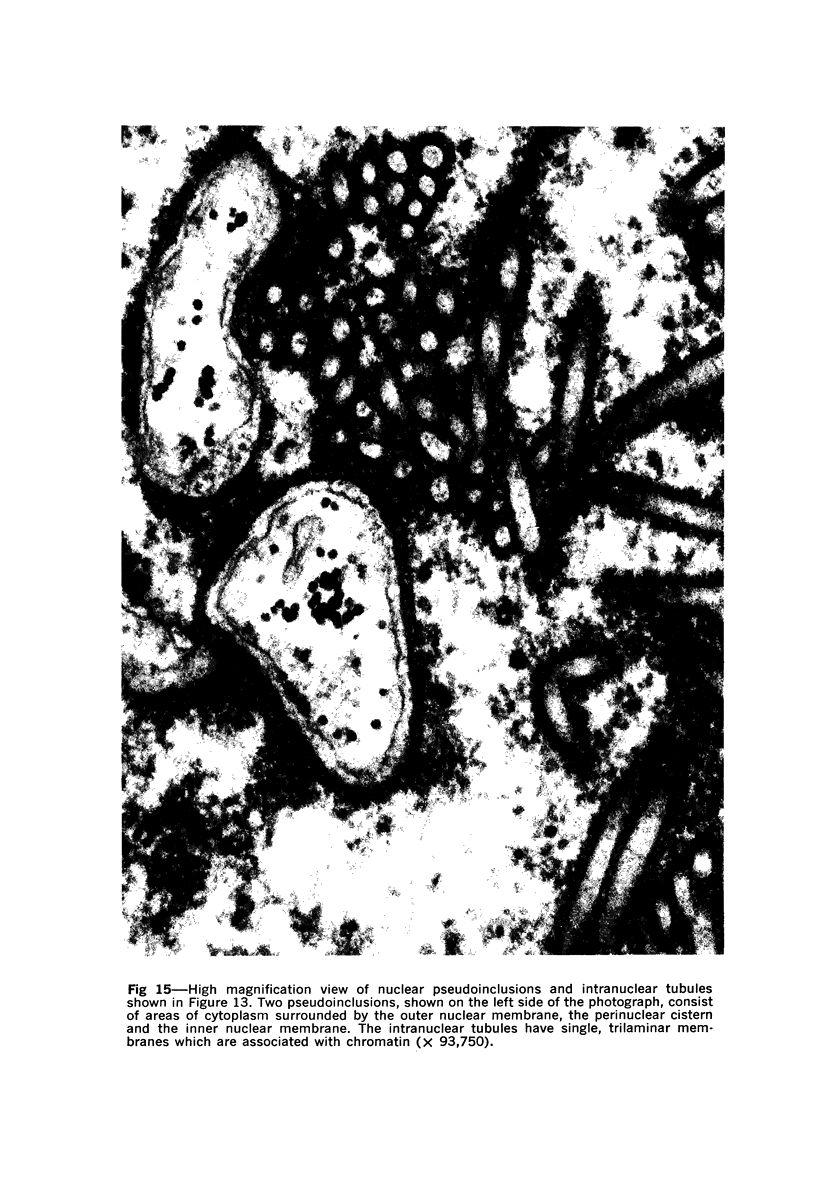
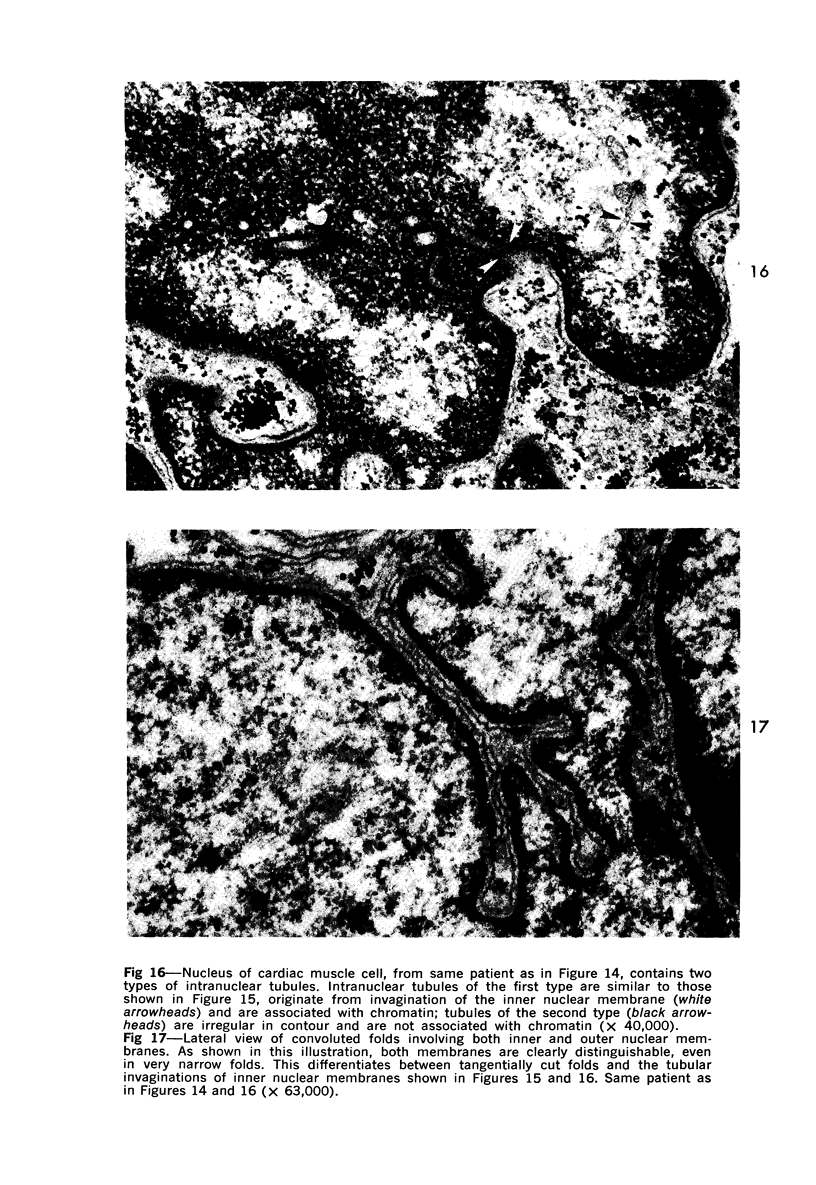
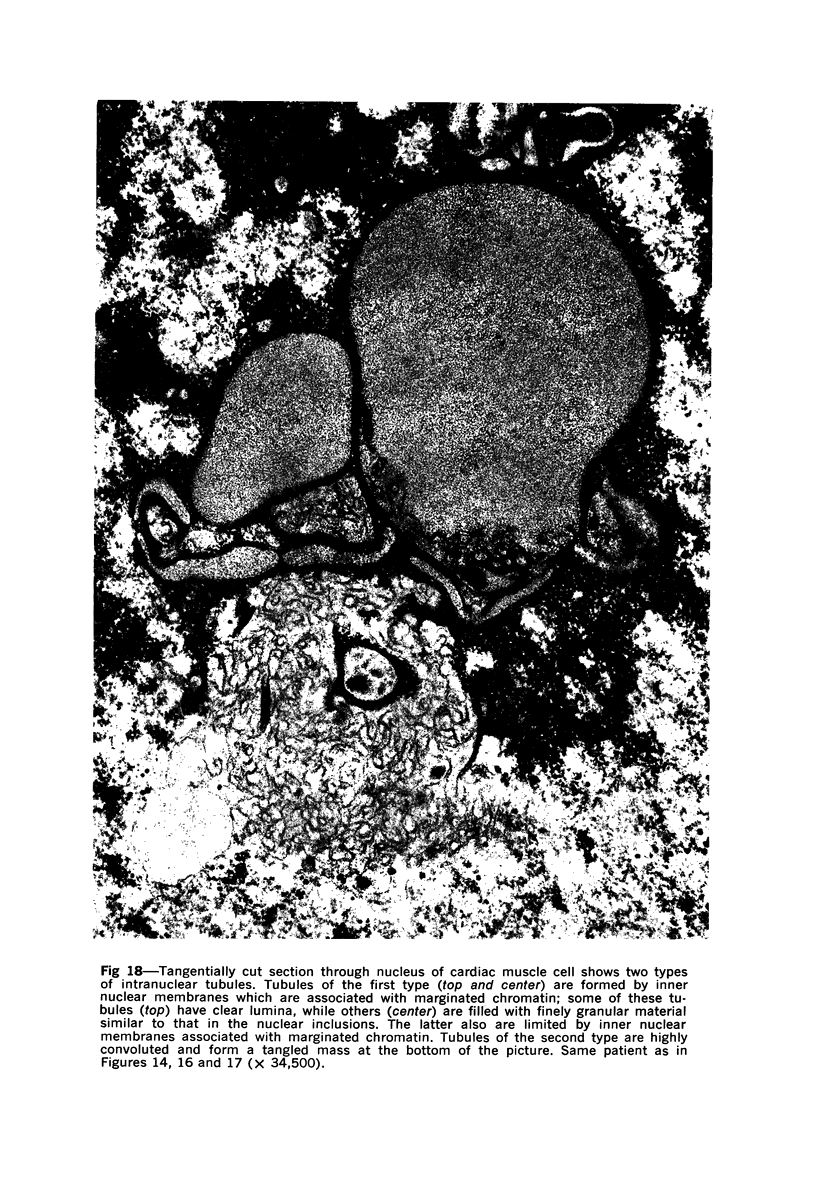
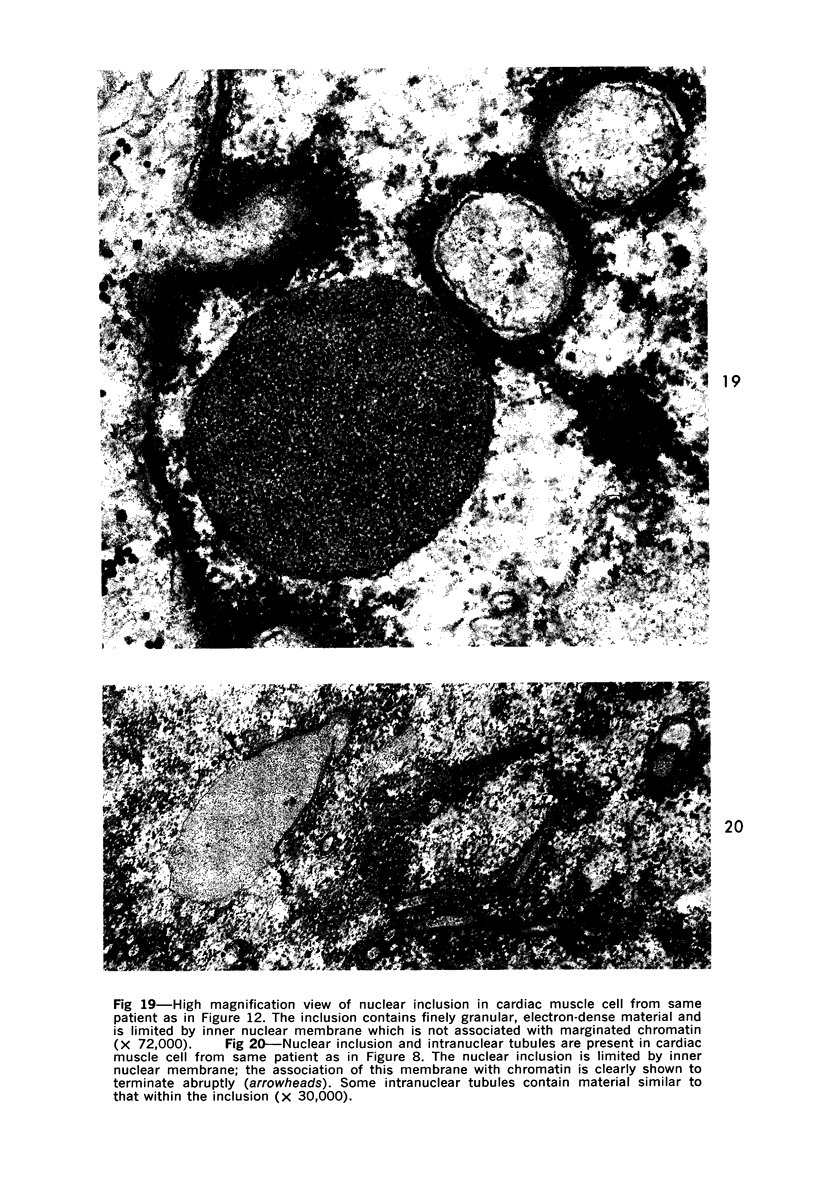
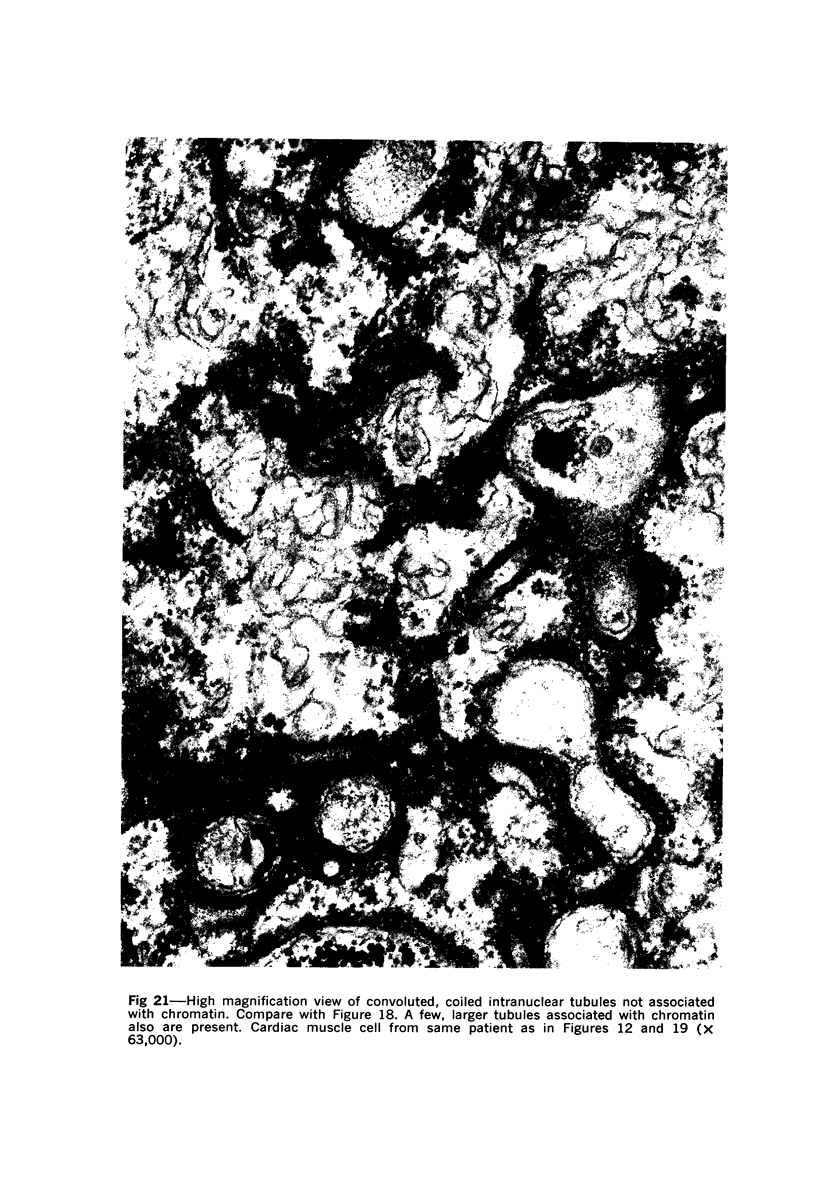
Nuclear membranes of cardiac muscle cells were studied in 134 patients with cardiac hypertrophy of various causes. Abnormalities observed consisted of: a) increased foldings and convolutions; b) nuclear pseudoinclusions formed by cytoplasmic organelles protruding into saccular invaginations of the nuclear membranes, and c) intranuclear tubules. The increased foldings and convolutions of the nuclear membranes and the nuclear pseudoinclusions appear to result from synthesis of nuclear membranes in excess of that needed to accommodate the increase in nuclear volume which occurs in hypertrophy. Intranuclear tubules were found in 6 patients and consisted of tubular invaginations, 400 to 650 A in diameter, of the inner nuclear membranes into the nucleoplasm. Some of these tubules were straight and cylindrical, and were associated with a peripheral layer of marginated chromatin; others were not associated with chromatin, appeared coiled and followed irregular courses. Intranuclear tubules in cardiac muscle cells probably represent an extreme cellular response to the stimulus of hypertrophy.
Full text
PDF






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong E. M., More I. A., McSeveny D., Chatfield W. R. Reappraisal of the ultrastructure of the human endometrial glandular cell. J Obstet Gynaecol Br Commonw. 1973 May;80(5):446–460. doi: 10.1111/j.1471-0528.1973.tb15961.x. [DOI] [PubMed] [Google Scholar]
- Babai F., Tremblay G., Dumont A. Intranuclear and intranucleolar tubular structures in Novikoff hepatoma cells. J Ultrastruct Res. 1969 Jul;28(1):125–130. doi: 10.1016/s0022-5320(69)90010-0. [DOI] [PubMed] [Google Scholar]
- Banker B. Q. A phase and electron microscopic study of dystrophic muscle. I. The pathological changes in the two-week-old Bar Harbor 129 dystrophic mouse. J Neuropathol Exp Neurol. 1967 Apr;26(2):259–275. doi: 10.1097/00005072-196704000-00006. [DOI] [PubMed] [Google Scholar]
- Bensch K. G., Malawista S. E. Microtubular crystals in mammalian cells. J Cell Biol. 1969 Jan;40(1):95–107. doi: 10.1083/jcb.40.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezesky I. K., Grimley P. M., Tyrrell S. A., Rabson A. S. Ultrastructure of a rat cytomegalovirus. Exp Mol Pathol. 1971 Jun;14(3):337–349. doi: 10.1016/0014-4800(71)90005-0. [DOI] [PubMed] [Google Scholar]
- Bloom S., Cancilla P. A. Conformational changes in myocardial nuclei of rats. Circ Res. 1969 Feb;24(2):189–196. doi: 10.1161/01.res.24.2.189. [DOI] [PubMed] [Google Scholar]
- Bloom S. Structural changes in nuclear envelopes during elongation of heart muscle cells. J Cell Biol. 1970 Jan;44(1):218–223. doi: 10.1083/jcb.44.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYMAN M. J. A new structure observed in the nucleolus of the human endometrial epithelial cell. Am J Obstet Gynecol. 1963 Jun 15;86:430–432. doi: 10.1016/0002-9378(63)90166-2. [DOI] [PubMed] [Google Scholar]
- COSSEL L. [Electron microscope findings on "inclusion bodies" in the nuclei of human liver epithelial cells]. Z Zellforsch Mikrosk Anat. 1962;57:512–519. [PubMed] [Google Scholar]
- Carlson E. C., Ollerich D. A. Intranuclear tubules in trophoblast 3 of rat and mouse chorioallantoic placenta. J Ultrastruct Res. 1969 Jul;28(1):150–160. doi: 10.1016/s0022-5320(69)90013-6. [DOI] [PubMed] [Google Scholar]
- Chandra S., Brown D. E., Aldenderfer P., Garon C., Buscheck F. T., Manaker R. A. Morphologic and serologic studies of transplanted human leukocyte culture (M-1) cells in laboratory animals. Cancer Res. 1969 Oct;29(10):1829–1839. [PubMed] [Google Scholar]
- Chandra S. Undulating tubules associated with endoplasmic reticulum in pathologic tissues. Lab Invest. 1968 Apr;18(4):422–428. [PubMed] [Google Scholar]
- Chopra H. C., Lloyd B. J., Jr, Ablashi D. V., Armstrong G. R. Morphologic studies of a cytomegalovirus isolated from an owl monkey. J Natl Cancer Inst. 1972 May;48(5):1333–1340. [PubMed] [Google Scholar]
- Chou S. M., Cherry J. D. Ultrastructure of Cowdry type A inclusions. 1. In human herpes simplex encephalitis. Neurology. 1967 Jun;17(6):575–passim. doi: 10.1212/wnl.17.6.575. [DOI] [PubMed] [Google Scholar]
- Chou S. M. Myxovirus-like structures and accompanying nuclear changes in chronic polymyositis. Arch Pathol. 1968 Dec;86(6):649–658. [PubMed] [Google Scholar]
- Chou S. M. Myxovirus-like structures in a case of human chronic polymyositis. Science. 1967 Dec 15;158(3807):1453–1455. doi: 10.1126/science.158.3807.1453. [DOI] [PubMed] [Google Scholar]
- DUBRAUSZKY V., POHLMANN G. [The ultra-structure of the corpus endometrium during the cycle]. Arch Gynakol. 1961;196:180–199. doi: 10.1007/BF00669449. [DOI] [PubMed] [Google Scholar]
- Dallner G., Bergstrand A., Nilsson R. Heterogeneity of rough-surfaced liver microsomal membranes of adult, phenobarbital-treated, and newborn rats. J Cell Biol. 1968 Aug;38(2):257–276. doi: 10.1083/jcb.38.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Granoff A., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. II. Ultrastructural studies and sequential development of virus isolated from normal and tumor tissue. Virology. 1966 May;29(1):149–156. doi: 10.1016/0042-6822(66)90204-2. [DOI] [PubMed] [Google Scholar]
- Emans J. B., Jones A. L. Hypertrophy of liver cell smooth surfaced reticulum following progesterone administration. J Histochem Cytochem. 1968 Sep;16(9):561–571. doi: 10.1177/16.9.561. [DOI] [PubMed] [Google Scholar]
- FALKE D., SIEGERT R., VOGELL W. [Electron microscopic findings on the problem of double membrane formation in herpes simplex virus]. Arch Gesamte Virusforsch. 1959;9:484–496. [PubMed] [Google Scholar]
- FAWCETT D. W. Electron microscope observations on intracellular virus-like particles associated with the cells of the Lucké renal adenocarcinoma. J Biophys Biochem Cytol. 1956 Nov 25;2(6):725–741. doi: 10.1083/jcb.2.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAJOLA W. J., GREIDER M. H., KLEINFELD R. G. Electron microscopy of intranuclear inclusions found in human and rat liver parenchymal cells. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):435–438. doi: 10.1083/jcb.2.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN J. A., SPURLOCK B. O. A new epoxy embedment for electron microscopy. J Cell Biol. 1962 Jun;13:437–443. doi: 10.1083/jcb.13.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W., McNutt N. S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969 Jul;42(1):1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans V. J., Massumi R. A., Shugoll G. I., Ali N., Roberts W. C. Ultrastructural studies of myocardial biopsies in 45 patients with obstructive or congestive cardiomyopathy. Recent Adv Stud Cardiac Struct Metab. 1973;2:231–272. [PubMed] [Google Scholar]
- Ferrans V. J., Morrow A. G., Roberts W. C. Myocardial ultrastructure in idiopathic hypertrophic subaortic stenosis. A study of operatively excised left ventricular outflow tract muscle in 14 patients. Circulation. 1972 Apr;45(4):769–792. doi: 10.1161/01.cir.45.4.769. [DOI] [PubMed] [Google Scholar]
- Ferrans V. J., Roberts W. C. Intermyofibrillar and nuclear-myofibrillar connections in human and canine myocardium. An ultrastructural study. J Mol Cell Cardiol. 1973 Jun;5(3):247–257. doi: 10.1016/0022-2828(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Fritzler M. J., Church R. B., Wagenaar E. B. Ultrastructure of taper hepatoma ascites cells. J Electron Microsc (Tokyo) 1973;22(1):73–90. [PubMed] [Google Scholar]
- GREGG M. B., MORGAN C. Reduplication of nuclear membranes in HeLa cells infected with adenovirus. J Biophys Biochem Cytol. 1959 Dec;6:539–540. doi: 10.1083/jcb.6.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouranton J. Development of an intranuclear nonoccluded rod-shaped virus in some midgut cells of an adult insect, Gyrinus natator L. (Coleoptera). J Ultrastruct Res. 1972 May;39(3):281–294. doi: 10.1016/s0022-5320(72)90023-8. [DOI] [PubMed] [Google Scholar]
- Györkey F., Sinkovics J. G., Min K. W., Györkey P. A morphologic study on the occurrence and distribution of structures resembling viral nucleocapsids in collagen diseases. Am J Med. 1972 Aug;53(2):148–158. doi: 10.1016/0002-9343(72)90125-8. [DOI] [PubMed] [Google Scholar]
- HOSHINO M. The deep invagination of the inner nuclear membrane into the nucleoplasm in the ascites hepatoma cells. Exp Cell Res. 1961 Sep;24:606–609. doi: 10.1016/0014-4827(61)90465-7. [DOI] [PubMed] [Google Scholar]
- HOWES E. L., Jr, PRICE H. M., BLUMBERG J. M. THE EFFECTS OF A DIET PRODUCING LIPOCHROME PIGMENT (CEROID) ON THE ULTRASTRUCTURE OF SKELETAL MUSCLE IN THE RAT. Am J Pathol. 1964 Oct;45:599–631. [PMC free article] [PubMed] [Google Scholar]
- Haas J. E., Yunis E. J. Viral crystalline arrays in human coxsackie myocarditis. Lab Invest. 1970 Oct;23(4):442–446. [PubMed] [Google Scholar]
- Harter D. H., Tellez-Nagel I. Attempts to isolate SSPE agent in cell culture. Neurology. 1968 Jan;18(1 Pt 2):133–137. doi: 10.1212/wnl.18.1_part_2.133. [DOI] [PubMed] [Google Scholar]
- Herndon R. M., Rubinstein L. J. Light and electron microscopy observations on the development of viral particles in the inclusions of Dawson's encephalitis (subacute sclerosing panencephalitis). Neurology. 1968 Jan;18(1 Pt 2):8–20. doi: 10.1212/wnl.18.1_part_2.008. [DOI] [PubMed] [Google Scholar]
- Hutterer F., Schaffner F., Klion F. M., Popper H. Hypertrophic, hypoactive smooth endoplasmic reticulum: a sensitive indicator of hepatotoxicity exemplified by dieldrin. Science. 1968 Sep 6;161(3845):1017–1019. doi: 10.1126/science.161.3845.1017. [DOI] [PubMed] [Google Scholar]
- Jensen A. B., Spjut H. J., Smith M. N., Rapp F. Intracellular branched tubular structures in osteosarcoma. An ultrastructural and serological study. Cancer. 1971 Jun;27(6):1440–1448. doi: 10.1002/1097-0142(197106)27:6<1440::aid-cncr2820270626>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Karasaki S. Passage of cytoplasmic lipid into interphase nuclei in preneoplastic rat liver. J Ultrastruct Res. 1973 Mar;42(5):463–478. doi: 10.1016/s0022-5320(73)80020-6. [DOI] [PubMed] [Google Scholar]
- Kohorn E. I., Rice S. I., Gordon M. In vitro production of nucleolar channel system by progesterone in human endometrium. Nature. 1970 Nov 14;228(5272):671–672. doi: 10.1038/228671a0. [DOI] [PubMed] [Google Scholar]
- Kohorn E. I., Rice S. I., Hemperly S., Gordon M. The relation of the structure of progestational steroids to nucleolar differentiation in human endometrium. J Clin Endocrinol. 1972;34:257–264. [PubMed] [Google Scholar]
- LEDUC E. H., WILSON J. W. An electron microscope study of intranuclear inclusions in mouse liver and hepatoma. J Biophys Biochem Cytol. 1959 Dec;6:427–430. doi: 10.1083/jcb.6.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuhl W. H. Electron microscopic study of the effects of tissue culture on human endometrium. Am J Obstet Gynecol. 1968 Sep 15;102(2):192–201. doi: 10.1016/0002-9378(68)90318-9. [DOI] [PubMed] [Google Scholar]
- MCGAVRAN M. H., SMITH M. G. ULTRASTRUCTURAL, CYTOCHEMICAL, AND MICROCHEMICAL OBSERVATIONS ON CYTOMEGALOVIRUS (SALIVARY GLAND VIRUS) INFECTION OF HUMAN CELLS IN TISSUE CULTURE. Exp Mol Pathol. 1965 Feb;76:1–10. doi: 10.1016/0014-4800(65)90019-5. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORICARD R., MORICARD F. MODIFICATIONS CYTOPLASMIQUES ET NUCL'EAIRES ULTRASTRUCTURALES UT'ERINES AU COURS DE L''ETAT FOLLICO-LUT'EINIQUE 'A GLYCOG'ENE MASSIF. (MITOCHONDRIES--GLYCOG'ENE--NUCL'EOLON'EMA--CHROMATINE SEXUELLE--FORMATIONS TUBALAIRES NUCL'EAIRES) Gynecol Obstet (Paris) 1964 Apr-May;63:203–220. [PubMed] [Google Scholar]
- Maron B. J., Ferrans V. J. Aggregates of tubules in human cardiac muscle cells. J Mol Cell Cardiol. 1974 Jun;6(3):249–264. doi: 10.1016/0022-2828(74)90054-6. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Ferrans V. J., Henry W. L., Clark C. E., Redwood D. R., Roberts W. C., Morrow A. G., Epstein S. E. Differences in distribution of myocardial abnormalities in patients with obstructive and nonobstructive asymmetric septal hypertrophy (ASH). Light and electron microscopic findings. Circulation. 1974 Sep;50(3):436–446. doi: 10.1161/01.cir.50.3.436. [DOI] [PubMed] [Google Scholar]
- Middelkamp J. N., Patrizi G., Reed C. A. Light and electron microscopic studies of the guinea pig cytomegalovirus. J Ultrastruct Res. 1967 Apr;18(1):85–101. doi: 10.1016/s0022-5320(67)80233-8. [DOI] [PubMed] [Google Scholar]
- More I. A., Armstrong E. M., McSeveney D., Chatfield W. R. The morphogenesis and fate of the nucleolar channel system in the human endometrial glandular cell. J Ultrastruct Res. 1974 Apr;47(20):74–85. doi: 10.1016/s0022-5320(74)90027-6. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B. A transplantable rat liver tumor induced by 4-dimethylaminoazobenzene. Cancer Res. 1957 Nov;17(10):1010–1027. [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Nakayama I., Nickerson P. A. Intranuclear inclusions in mammotrophs of the female Mongolian gerbil. Am J Anat. 1972 Sep;135(1):93–104. doi: 10.1002/aja.1001350108. [DOI] [PubMed] [Google Scholar]
- Nickerson P. A. Induction of intranuclear inclusions by estrogen in mammotrophs of the Mongolian gerbil. J Ultrastruct Res. 1973 Jul;44(1):41–48. doi: 10.1016/s0022-5320(73)90039-7. [DOI] [PubMed] [Google Scholar]
- Nieland N. W., Hashimoto K., Masi A. T. Microtubular inclusions in normal skin of systemic lupus erythematosus patients. Arthritis Rheum. 1972 Mar-Apr;15(2):193–200. doi: 10.1002/art.1780150210. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. Mitochondrial and nuclear interaction. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):351–352. doi: 10.1083/jcb.2.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRIZI G., MIDDELKAMP J. N., HERWEG J. C., THORNTON H. K. HUMAN CYTOMEGALOVIRUS: ELECTRON MICROSCOPY OF A PRIMARY VIRAL ISOLATE. J Lab Clin Med. 1965 May;65:825–838. [PubMed] [Google Scholar]
- Patrizi G., Middelkamp J. N., Reed C. A. Reduplication of nuclear membranes in tissue-culture cells infected with guinea-pig cytomegalovirus. Am J Pathol. 1967 May;50(5):779–790. [PMC free article] [PubMed] [Google Scholar]
- Périer O., Vanderhaeghen J. J. Indications étiologiques apportées par la microscopie électronique dans certaines encéphalites humaines. Rev Neurol (Paris) 1966 Aug;115(2):250–254. [PubMed] [Google Scholar]
- ROBERTSON D. M. ELECTRON MICROSCOPIC STUDIES OF NUCLEAR INCLUSIONS IN MENINGIOMAS. Am J Pathol. 1964 Nov;45:835–848. [PMC free article] [PubMed] [Google Scholar]
- RUEBNER B. H., HIRANO T., SLUSSER R. J., MEDEARIS D. N., Jr HUMAN CYTOMEGALOVIRUS INFECTION. ELECTRON MICROSCOPIC AND HISTOCHEMICAL CHANGES IN CULTURES OF HUMAN FIBROBLASTS. Am J Pathol. 1965 Mar;46:477–496. [PMC free article] [PubMed] [Google Scholar]
- RUEBNER B. H., MIYAI K., SLUSSER R. J., WEDEMEYER P., MEDEARIS D. N., Jr MOUSE CYTOMEGALOVIRUS INFECTION. AN ELECTRON MICROSCOPIC STUDY OF HEPATIC PARENCHYMAL CELLS. Am J Pathol. 1964 May;44:799–821. [PMC free article] [PubMed] [Google Scholar]
- Raine C. S., Fields B. N. Neurotropic virus-host relationship alterations due to variation in viral genome as studied by electron microscopy. Am J Pathol. 1974 Apr;75(1):119–138. [PMC free article] [PubMed] [Google Scholar]
- Robertson D. M., MacLean J. D. Nuclear inclusions in malignant gliomas. Arch Neurol. 1965 Sep;13(3):287–296. doi: 10.1001/archneur.1965.00470030067006. [DOI] [PubMed] [Google Scholar]
- Schaff Z., Heine U., Dalton A. J. Ultramorphological and ultracytochemical studies on tubuloreticular structures in lymphoid cells. Cancer Res. 1972 Dec;32(12):2696–2706. [PubMed] [Google Scholar]
- Schmitt D., Thivolet J., Perrot H., Tack J. L., Germain D. Aspects ultrastructuraux des inclusions de type viral dans les lésions cutaneés du lupus aigu et du lupus discoïde fix. Arch Dermatol Forsch. 1971;241(4):317–328. [PubMed] [Google Scholar]
- Shipkey F. H., Erlandson R. A., Bailey R. B., Babcock V. I., Southam C. M. Virus biographies. II. Growth of herpes simplex virus in tissue culture. Exp Mol Pathol. 1967 Feb;6(1):39–67. doi: 10.1016/0014-4800(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Sobel H. J. Cytoplasmic invaginations resembling nuclear inclusions. Arch Pathol. 1968 Jan;85(1):114–115. [PubMed] [Google Scholar]
- Sobel H. J., Schwarz R., Marquet E. Nonviral nuclear inclusions. I. Cytoplasmic invaginations. Arch Pathol. 1969 Feb;87(2):179–192. [PubMed] [Google Scholar]
- Stackpole C. W., Mizell M. Electron microscopic observations on herpes-type virus-related structures in the frog renal adenocarcinoma. Virology. 1968 Sep;36(1):63–72. doi: 10.1016/0042-6822(68)90117-7. [DOI] [PubMed] [Google Scholar]
- Tellez-Negal I., Harter D. H. Subacute sclerosing leukoencephalitis: ultrastructure of intranuclear and intracytoplasmic inclusions. Science. 1966 Nov 18;154(3751):899–901. doi: 10.1126/science.154.3751.899. [DOI] [PubMed] [Google Scholar]
- Terzakis J. A. The nucleolar channel system of human endometrium. J Cell Biol. 1965 Nov;27(2):293–304. doi: 10.1083/jcb.27.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzman B. G., Saito H., Kasac M. Tubular arrays in the endoplasmic reticulum in human tumor cells. Lab Invest. 1971 Jun;24(6):492–498. [PubMed] [Google Scholar]
- WEBER A., WHIPP S., USENIK E., FROMMES S. STRUCTURAL CHANGES IN THE NUCLEAR BODY IN THE ADRENAL ZONA FASCICULATA OF THE CALF FOLLOWING THE ADMINISTRATION OF ACTH. J Ultrastruct Res. 1964 Dec;11:564–576. doi: 10.1016/s0022-5320(64)80082-4. [DOI] [PubMed] [Google Scholar]
- Willson N. J., Schneider J. F., Rosen M., Belisle E. H. Ultrastructural pathology of murine cytomegalovirus infection in cultured mouse nervous system tissue. Am J Pathol. 1974 Mar;74(3):467–480. [PMC free article] [PubMed] [Google Scholar]