Abstract
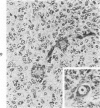
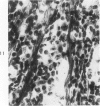
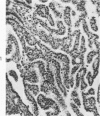
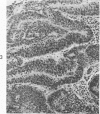
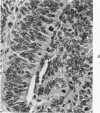
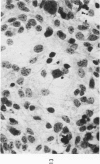
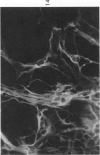
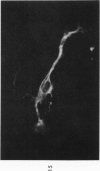
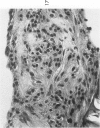
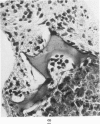
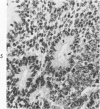
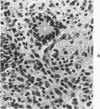
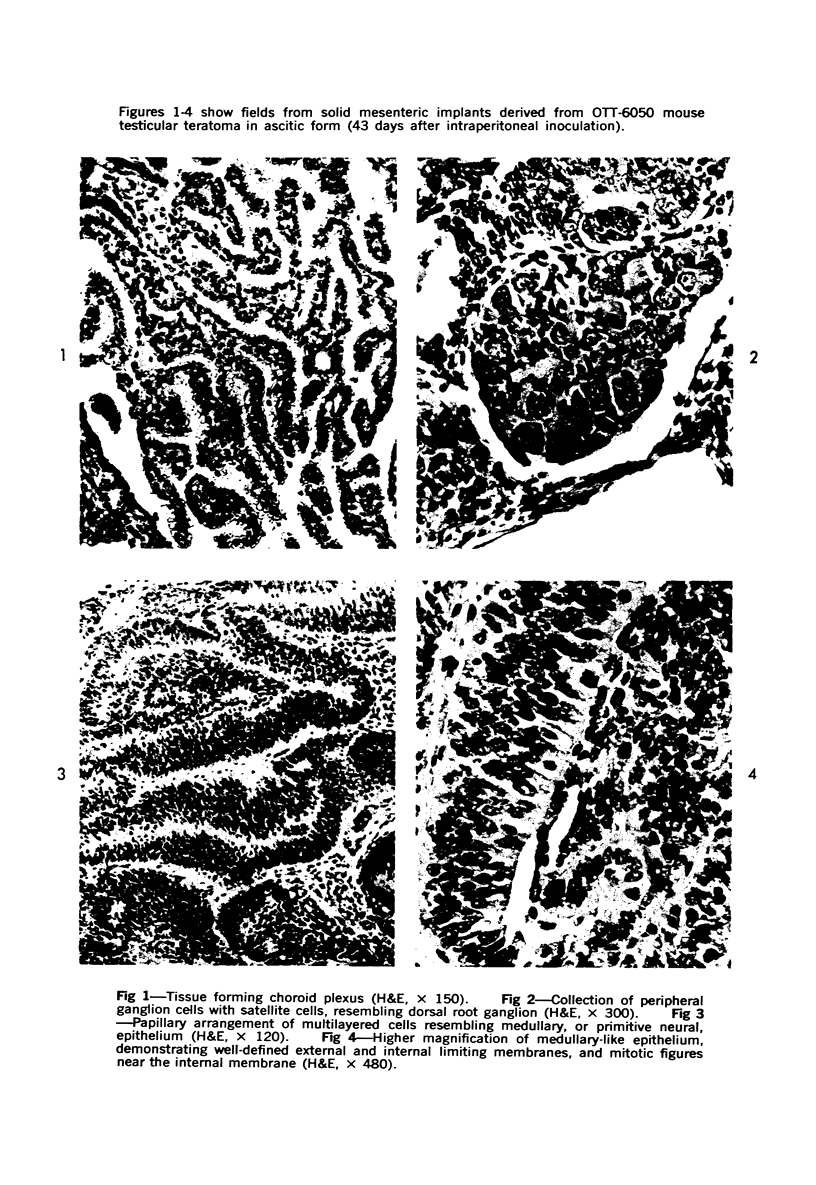
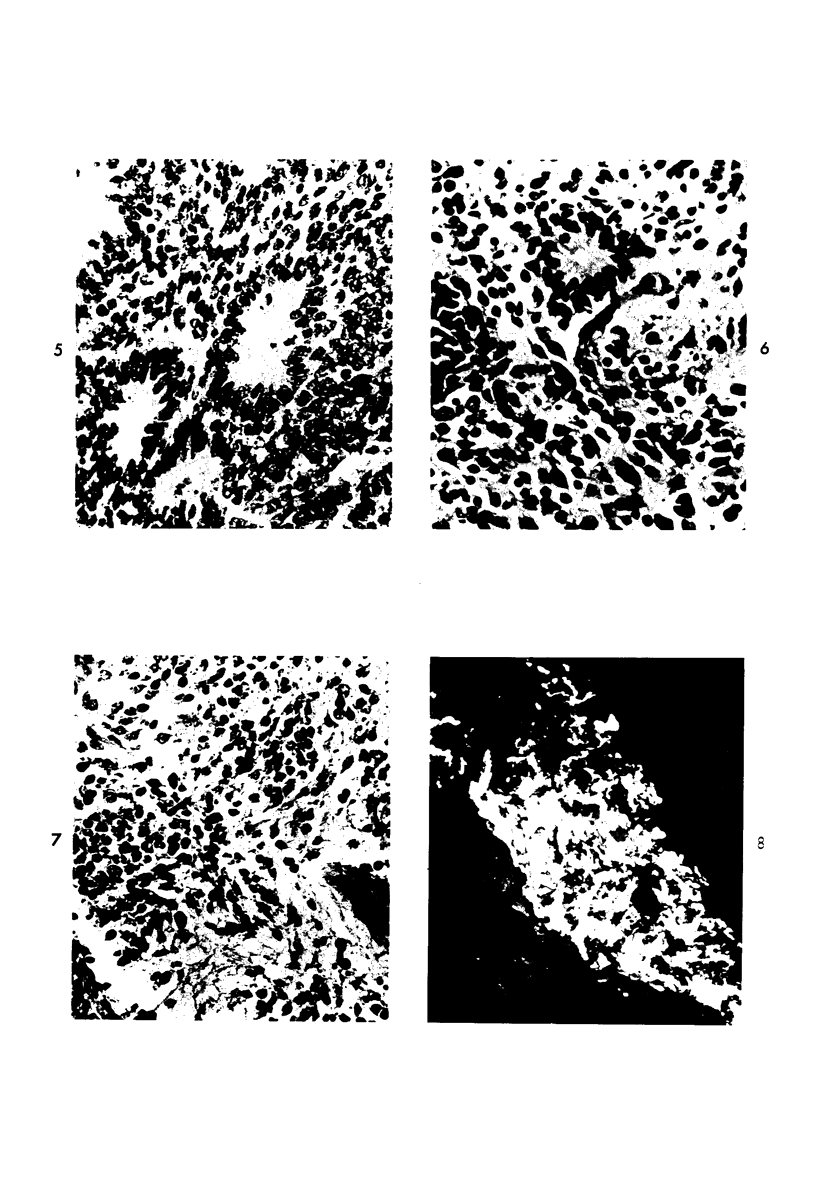
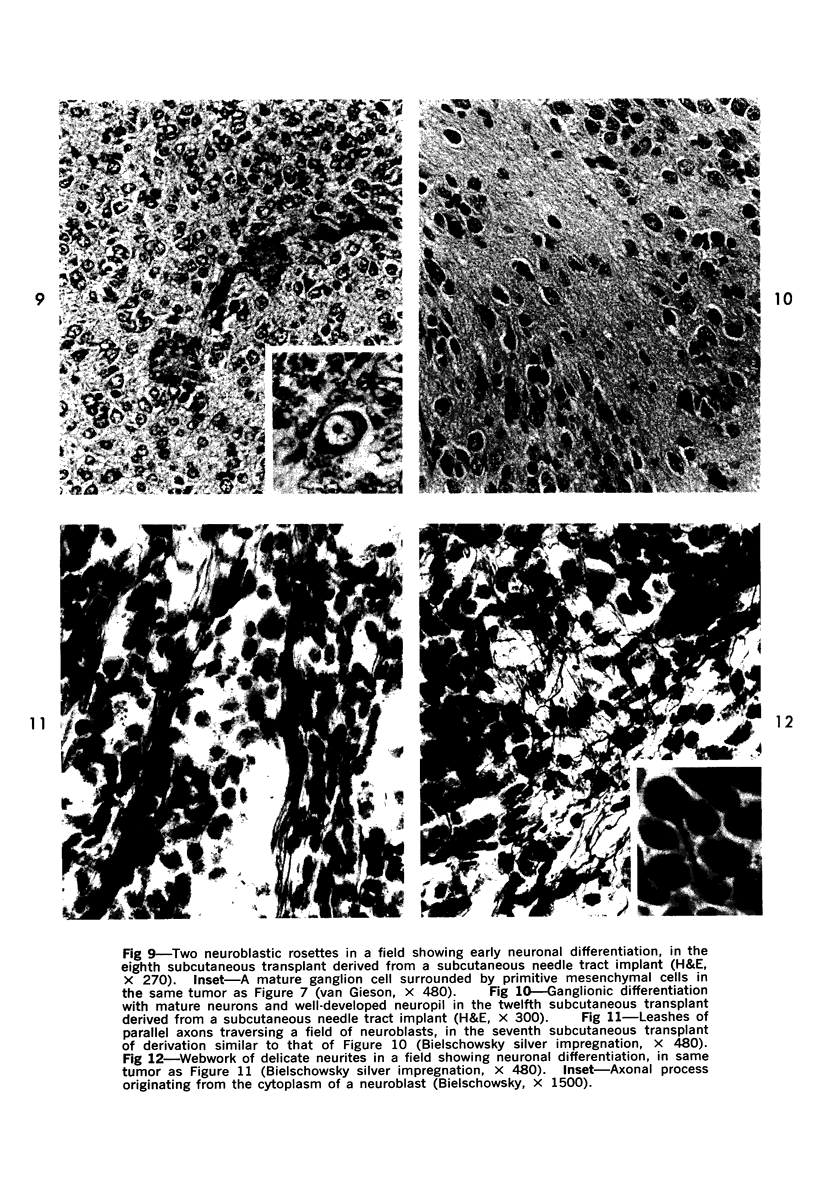
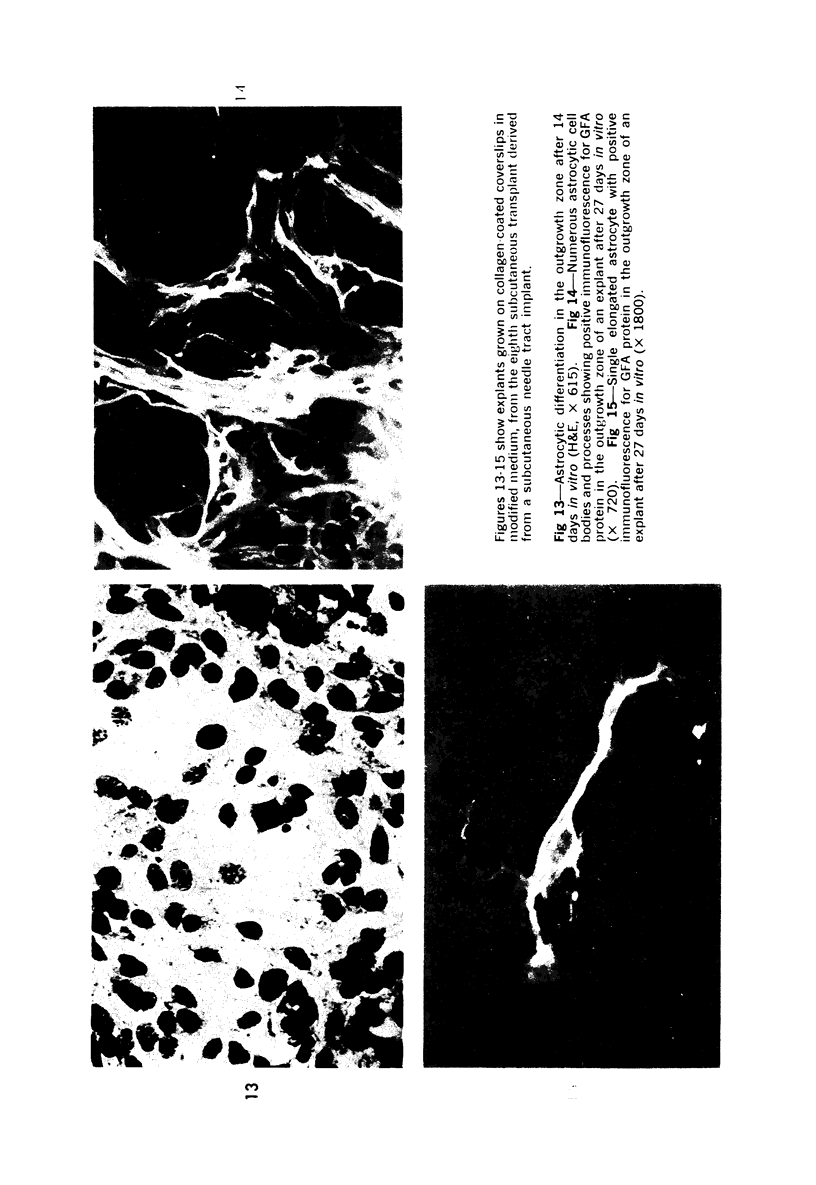
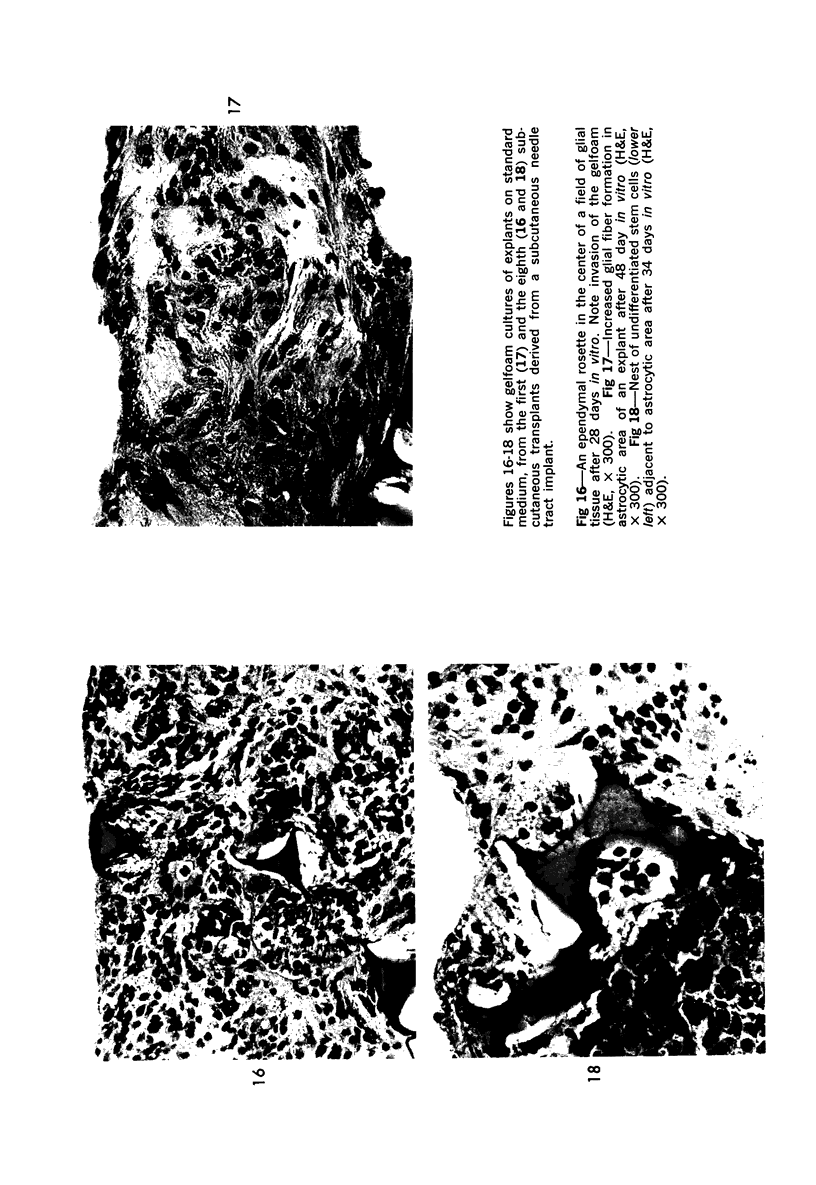
The various stages of divergent neuroepithelial differentiation were studied in the solid transplants of a transplantable mouse testicular teratoma (OTT-6050) maintained in both ascitic and solid forms. They included: a) areas of undifferentiated medullary epithelium corresponding to the rare human medulloepithelioma; b) areas of neuroblastic differentiation corresponding to neuroblastoma, with more mature neuronal differentiation corresponding to ganglioneuroma or, when mixed with glial elements, to ganglioglioma; and c) more mature neuroglial areas resembling astrocytoma, oligodendroglioma or ependymoma, as well as more primitive areas corresponding to ependymoblastoma. In tissue culture using collagen-coated coverslips, astrocytic differentiation was found in the outgrowth zone after 15 days, confirmed by immunofluorescence with antibodies to an astroglia-specific protein. In organ culture systems, glial components, including ependymal structures, were preserved in tumor explants, and astrocytic differentiation, as expressed by glial fiber formation, was increased after 4 to 6 weeks in vitro. No neuronal differentiation was demonstrable, however. The neuroepithelial component of this experimental teratoma may provide a model for the study of neoplastic neuroepithelial differentiation.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K., Bennett D. A genetically caused embryonal ectodermal tumor in the mouse. J Natl Cancer Inst. 1972 Jan;48(1):141–158. [PubMed] [Google Scholar]
- Bignami A., Dahl D. Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol. 1974 Jan 1;153(1):27–38. doi: 10.1002/cne.901530104. [DOI] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Damjanov I., Solter D., Belicza M., Skreb N. Teratomas obtained through extrauterine growth of seven-day mouse embryos. J Natl Cancer Inst. 1971 Mar;46(3):471–passim. [PubMed] [Google Scholar]
- Damjanov I., Solter D., Serman D. Teratocarcinoma with the capacity for differentiation restricted to neuro-ectodermal tissue. Virchows Arch B Cell Pathol. 1973 Jul 22;13(3):179–195. doi: 10.1007/BF02889307. [DOI] [PubMed] [Google Scholar]
- Deck J. H. Cerebral medulloepithelioma with maturation into ependymal cells and ganglion cells. J Neuropathol Exp Neurol. 1969 Jul;28(3):442–454. doi: 10.1097/00005072-196907000-00006. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Evans M. J. The isolation and properties of a clonal tissue culture strain of pluripotent mouse teratoma cells. J Embryol Exp Morphol. 1972 Aug;28(1):163–176. [PubMed] [Google Scholar]
- FAWCETT D. W. Bilateral ovarian teratomas in a mouse. Cancer Res. 1950 Nov;10(11):705–707. [PubMed] [Google Scholar]
- FEKETE E., FERRIGNO M. A. Studies on a transplantable teratoma of the mouse. Cancer Res. 1952 Jun;12(6):438–440. [PubMed] [Google Scholar]
- Jami J., Ritz E. Multipotentiality of single cells of transplantable teratocarcinomas derived from mouse embryo grafts. J Natl Cancer Inst. 1974 May;52(5):1547–1552. doi: 10.1093/jnci/52.5.1547. [DOI] [PubMed] [Google Scholar]
- Jellinger K. Cerebral medulloepithelioma. Acta Neuropathol. 1972;22(2):95–101. doi: 10.1007/BF00688777. [DOI] [PubMed] [Google Scholar]
- KLEINSMITH L. J., PIERCE G. B., Jr MULTIPOTENTIALITY OF SINGLE EMBRYONAL CARCINOMA CELLS. Cancer Res. 1964 Oct;24:1544–1551. [PubMed] [Google Scholar]
- Kahan B. W., Ephrussi B. Developmental potentialities of clonal in vitro cultures of mouse testicular teratoma. J Natl Cancer Inst. 1970 May;44(5):1015–1036. [PubMed] [Google Scholar]
- Karch S. B., Urich H. Medulloepithelioma: definition of an entity. J Neuropathol Exp Neurol. 1972 Jan;31(1):27–53. [PubMed] [Google Scholar]
- LAPHAM L. W., JOHNSTONE M. A., BRUNDJAR K. H. A NEW PARAFFIN METHOD FOR THE COMBINED STAINING OF MYELIN AND GLIAL FIBERS. J Neuropathol Exp Neurol. 1964 Jan;23:156–160. [PubMed] [Google Scholar]
- LEIGHTON J. The growth patterns of some transplantable animal tumors in sponge matrix tissue culture. J Natl Cancer Inst. 1954 Oct;15(2):275–293. [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Torosian M., Sarokhan A. J., Teresky A. K. Biochemical criteria for the in vitro differentiation of embryoid bodies produced by a transplantable teratoma of mice. The production of acetylcholine esterase and creatine phosphokinase by teratoma cells. J Cell Physiol. 1974 Oct;84(2):311–317. doi: 10.1002/jcp.1040840217. [DOI] [PubMed] [Google Scholar]
- Meier H., Myers D. D., Fox R. R., Laird C. W. Occurrence, pathological features, and propagation of gonadal teratomas in inbred mice and in rabbits. Cancer Res. 1970 Jan;30(1):30–34. [PubMed] [Google Scholar]
- Mukai N., Kobayashi S. Human adenovirus-induced medulloepitheliomatous neoplasms in Sprague-Dawley rats. Am J Pathol. 1973 Dec;73(3):671–690. [PMC free article] [PubMed] [Google Scholar]
- Mukai N., Kobayashi S. Primary brain and spinal cord tumors induced by human adenovirus type 12 in hamsters. J Neuropathol Exp Neurol. 1973 Oct;32(4):523–541. doi: 10.1097/00005072-197310000-00004. [DOI] [PubMed] [Google Scholar]
- PIERCE G. B., DIXON F. J., Jr Testicular teratomas. I. Demonstration of teratogenesis by metamorphosis of multipotential cells. Cancer. 1959 May-Jun;12(3):573–583. doi: 10.1002/1097-0142(195905/06)12:3<573::aid-cncr2820120316>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- PIERCE G. B., Jr, DIXON F. J., Jr, VERNEY E. L. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Lab Invest. 1960 Nov-Dec;9:583–602. [PubMed] [Google Scholar]
- PIERCE G. B., Jr, VERNEY E. L. An in vitro and in vivo study of differentiation in teratocarcinomas. Cancer. 1961 Sep-Oct;14:1017–1029. doi: 10.1002/1097-0142(196109/10)14:5<1017::aid-cncr2820140516>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Rosenthal M. D., Wishnow R. M., Sato G. H. In vitro growth and differetiation of clonal populations of multipotential mouse clls derived from a transplantable testicular teratocarcinoma. J Natl Cancer Inst. 1970 May;44(5):1001–1014. [PubMed] [Google Scholar]
- Rubinstein L. J., Herman M. M., Foley V. L. In vitro characteristics of human glioblastomas maintained in organ culture systems. Light microscopy observations. Am J Pathol. 1973 Apr;71(1):61–80. [PMC free article] [PubMed] [Google Scholar]
- Rubinstein L. J. Presidential address. Cytogenesis and differentiation of primitive central neuroepithelial tumors. J Neuropathol Exp Neurol. 1972 Jan;31(1):7–26. doi: 10.1097/00005072-197201000-00002. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J. The definition of the ependymoblastoma. Arch Pathol. 1970 Jul;90(1):35–45. [PubMed] [Google Scholar]
- STEVENS L. C. EXPERIMENTAL PRODUCTION OF TESTICULAR TERATOMAS IN MICE. Proc Natl Acad Sci U S A. 1964 Sep;52:654–661. doi: 10.1073/pnas.52.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS L. C. Embryology of testicular teratomas in strain 129 mice. J Natl Cancer Inst. 1959 Dec;23:1249–1295. [PubMed] [Google Scholar]
- STEVENS L. C. Embryonic potency of embryoid bodies derived from a transplantable testicular teratoma of the mouse. Dev Biol. 1960 Jun;2:285–297. doi: 10.1016/0012-1606(60)90010-5. [DOI] [PubMed] [Google Scholar]
- STEVENS L. C., HUMMEL K. P. A description of spontaneous congenital testicular teratomas in strain 129 mice. J Natl Cancer Inst. 1957 May;18(5):719–747. [PubMed] [Google Scholar]
- STEVENS L. C. Studies on transplantable testicular teratomas of strain 129 mice. J Natl Cancer Inst. 1958 Jun;20(6):1257–1275. doi: 10.1093/jnci/20.6.1257. [DOI] [PubMed] [Google Scholar]
- Sipe J. C., Herman M. M., Rubinstein L. J. Electron microscopic observations on human glioblastomas and astrocytomas maintained in organ culture systems. Am J Pathol. 1973 Dec;73(3):589–606. [PMC free article] [PubMed] [Google Scholar]
- Sipe J. C., Rubinstein L. J., Herman M. M., Bignami A. Ethylnitrosourea-induced astrocytomas. Morphologic observations on rat tumors maintained in tissue and organ culture systems. Lab Invest. 1974 Dec;31(6):571–579. [PubMed] [Google Scholar]
- Stevens L. C. A new inbred subline of mice (129-terSv) with a high incidence of spontaneous congenital testicular teratomas. J Natl Cancer Inst. 1973 Jan;50(1):235–242. doi: 10.1093/jnci/50.1.235. [DOI] [PubMed] [Google Scholar]
- Stevens L. C. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Dev Biol. 1970 Mar;21(3):364–382. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- Stevens L. C., Varnum D. S. The development of teratomas from parthenogenetically activated ovarian mouse eggs. Dev Biol. 1974 Apr;37(2):369–380. doi: 10.1016/0012-1606(74)90155-9. [DOI] [PubMed] [Google Scholar]
- THIERY M. OVARIAN TERATOMA IN THE MOUSE. Br J Cancer. 1963 Jun;17:231–234. doi: 10.1038/bjc.1963.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teresky A. K., Marsden M., Kuff E. L., Levine A. J. Morphological criteria for the in vitro differentiation of embryoid bodies produced by a transplantable teratoma of mice. J Cell Physiol. 1974 Oct;84(2):319–332. doi: 10.1002/jcp.1040840218. [DOI] [PubMed] [Google Scholar]
- Uyeda C. T., Eng L. F., Bignami A. Immunological study of the glial fibrillary acidic protein. Brain Res. 1972 Feb 11;37(1):81–89. doi: 10.1016/0006-8993(72)90347-2. [DOI] [PubMed] [Google Scholar]