Abstract
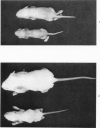
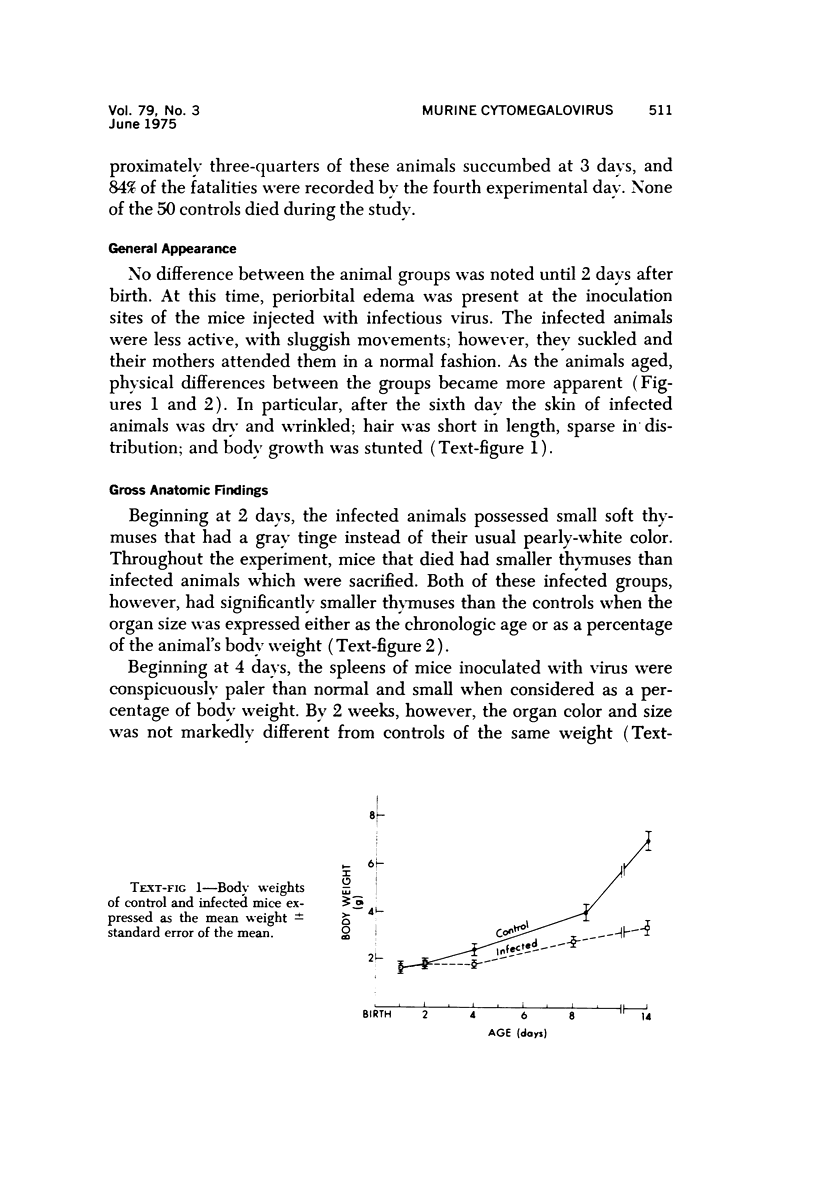
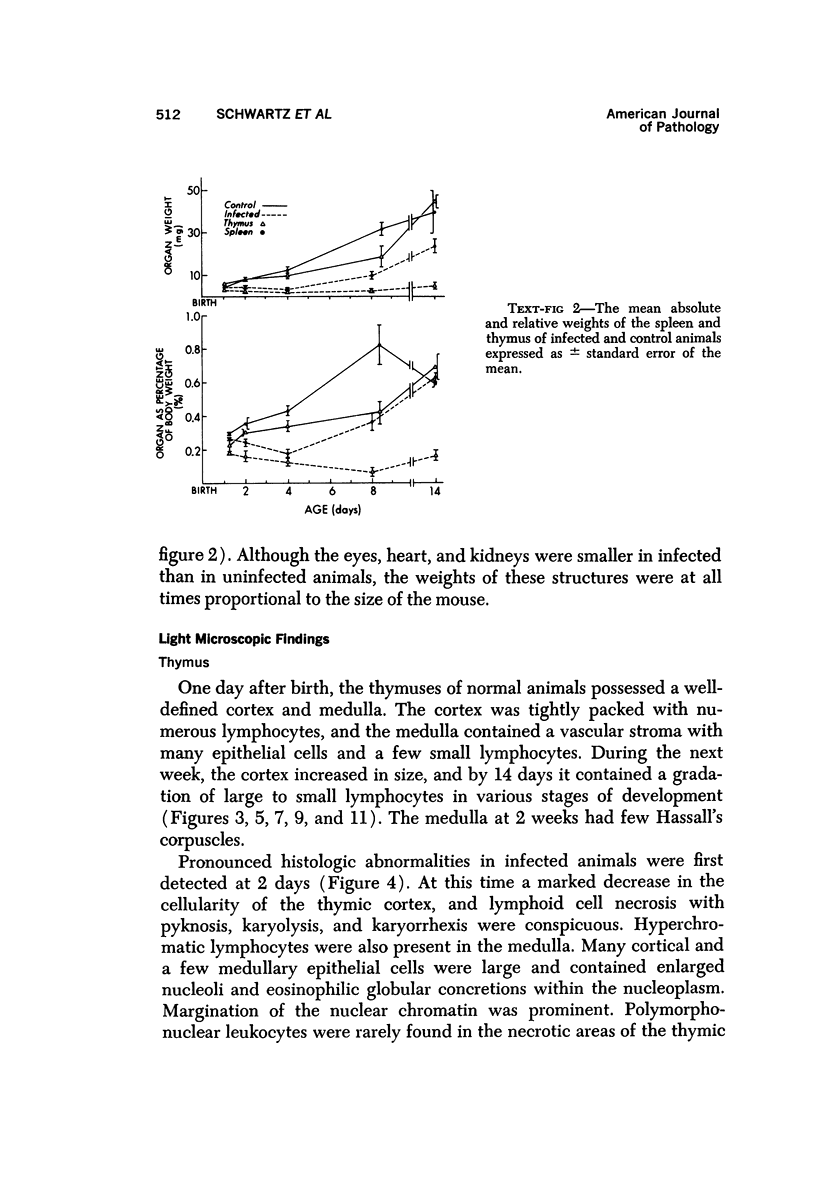
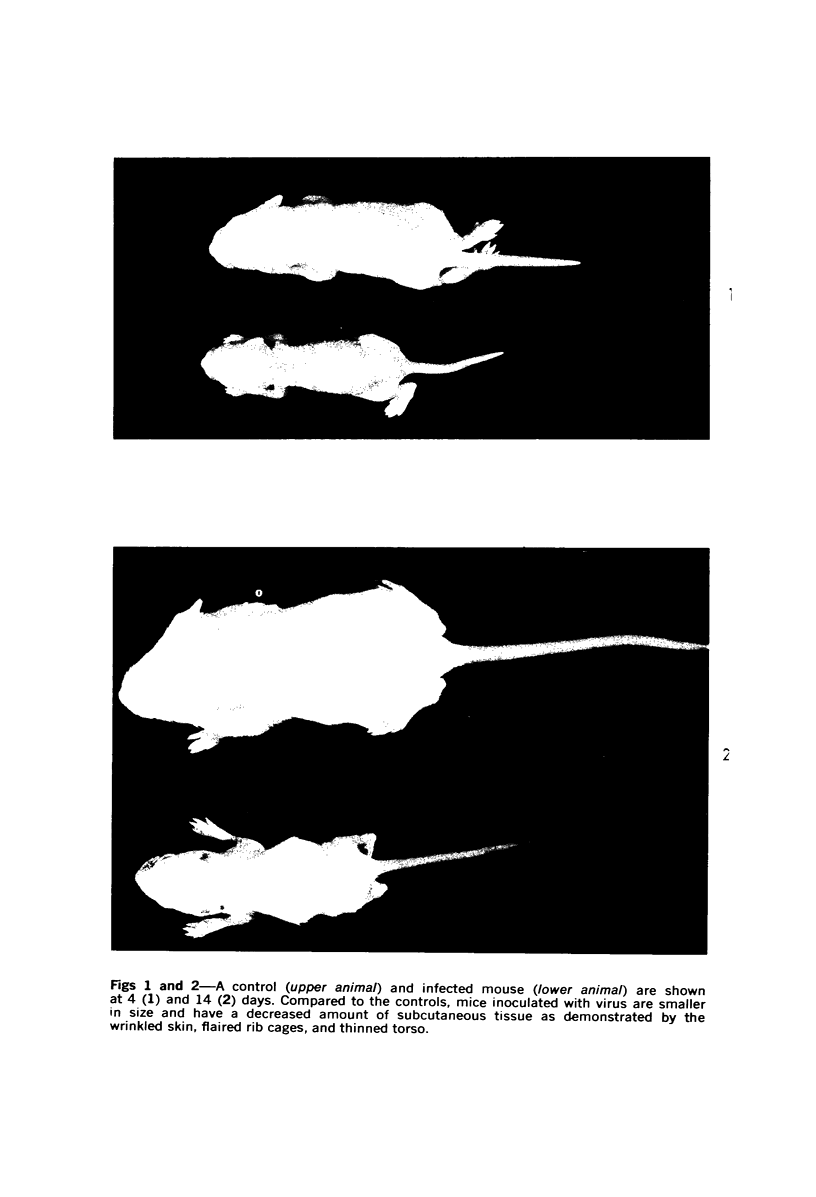
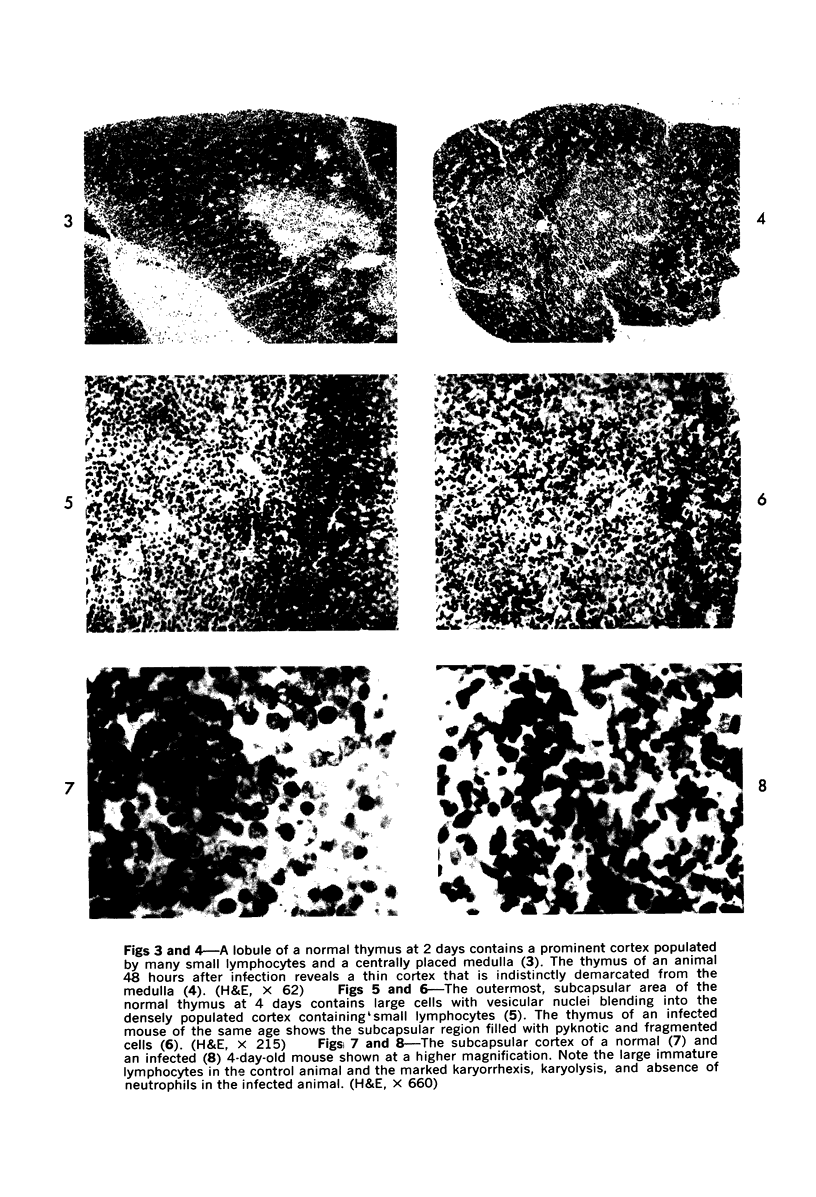
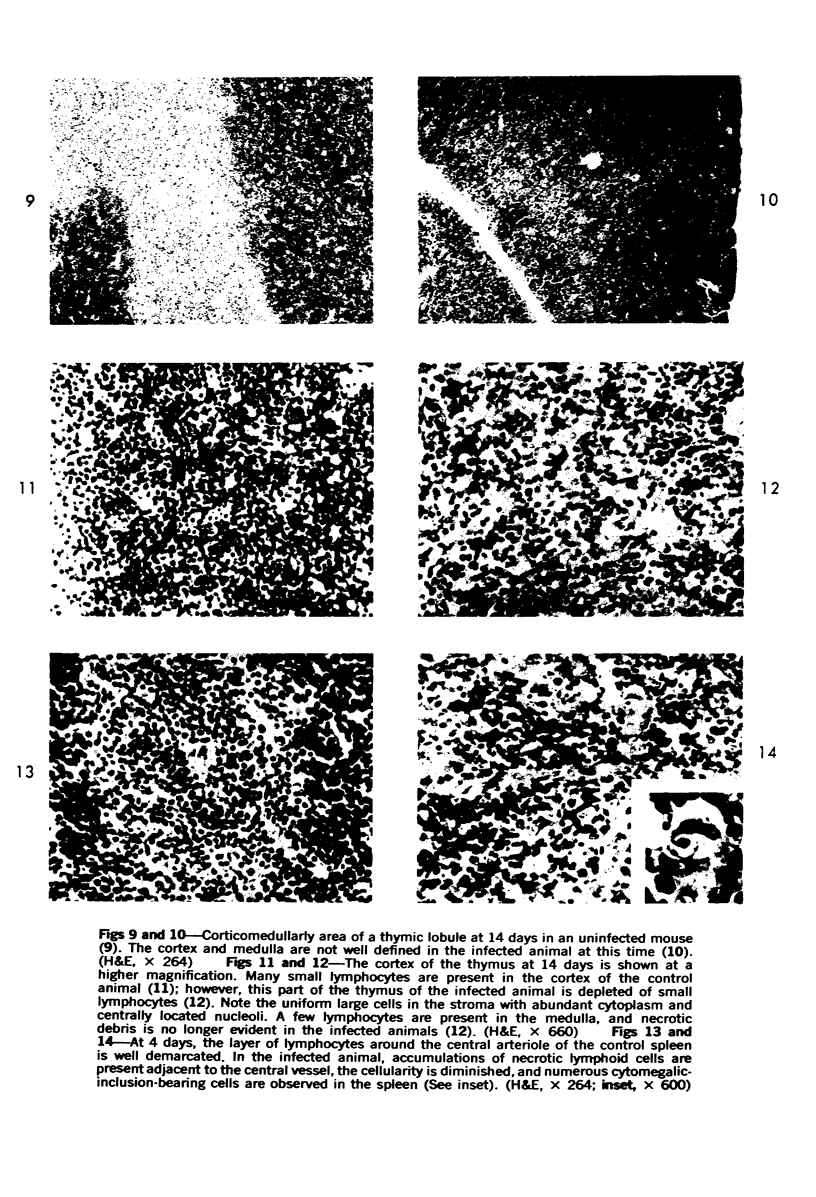
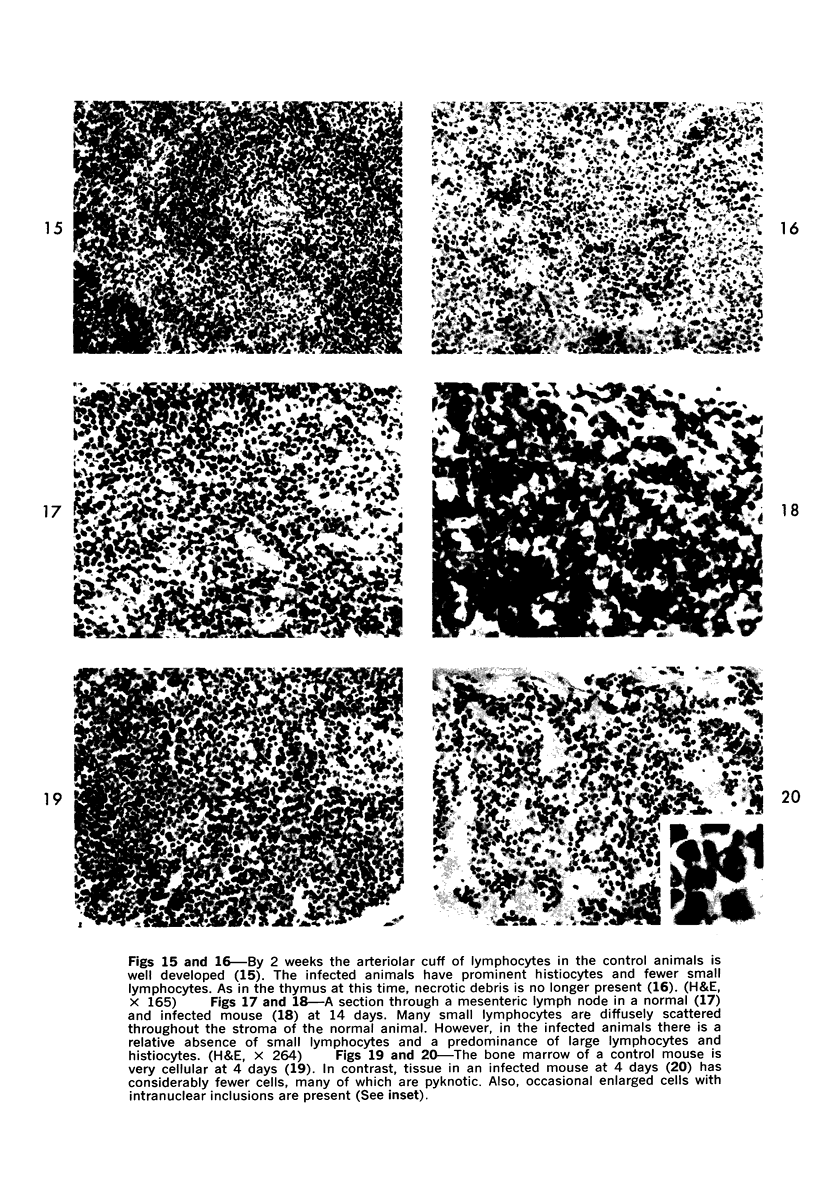
During studies on the effect of murine cytomegalovirus on the developing retina, virus was inoculated into the eyes of newborn Swiss mice, and the animals were sacrificed at various times thereafter. Controls consisted of mice inoculated with ultraviolet-inactivated murine cytomegalovirus and uninjected mice. Marked lymphoid cell necrosis, thymic atrophy, pronounced growth retardation, bacteremia, and death occurred in the animals inoculated with live virus. this virus-induced injury resulted in a marked depletion of lymphocytes in the subcapsular and cortical areas of the thymus as well as in the spleen, lymph nodes, and Peyer's patches. Areas of necrosis with viral inclusions were present at the site of inoculation and in various other organs including the spleen and bone marrow. Since growth retardation has been associated with thymic atrophy due to other causes, the observed abnormal physical development in the present study was interpreted as a sequel to the thymic injury. An implication of this study is that some human infants with concomitant immune deficiency and viral infection may have a primary viral disease with resultant secondary lymphoid tissue alterations, rather than a thymic disorder with a subsequent viral infection.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZAR H. A. BACTERIAL INFECTION AND WASTING IN NEONATALLY THYMECTOMIZED RATS. Proc Soc Exp Biol Med. 1964 Jul;116:817–823. [PubMed] [Google Scholar]
- BRODSKY I., ROWE W. P. Chronic subclinical infection with mouse salivary gland virus. Proc Soc Exp Biol Med. 1958 Dec;99(3):654–655. doi: 10.3181/00379727-99-24451. [DOI] [PubMed] [Google Scholar]
- BROOKE M. S. EXPERIMENTAL RUNT DISEASE IN MICE CAUSED BY SALMONELLA TYPHIMURIUM, VAR. COPENHAGEN. J Exp Med. 1964 Sep 1;120:375–387. doi: 10.1084/jem.120.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benirschke K., Mendoza G. R., Bazeley P. L. Placental and fetal manifestations of cytomegalovirus infection. Virchows Arch B Cell Pathol. 1974;16(2):121–139. doi: 10.1007/BF02894070. [DOI] [PubMed] [Google Scholar]
- De Sousa M. A., Parrott D. M., Pantelouris E. M. The lymphoid tissues in mice with congenital aplasia of the thymus. Clin Exp Immunol. 1969 Jun;4(6):637–644. [PMC free article] [PubMed] [Google Scholar]
- Garcia A. G., Olinto F., Fortes T. G. Thymic hypoplasia due to congenital rubella. Arch Dis Child. 1974 Mar;49(3):181–185. doi: 10.1136/adc.49.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar J. L. Light and electron microscopy of the human fetal thymus. Anat Rec. 1974 Aug;179(4):463–476. doi: 10.1002/ar.1091790406. [DOI] [PubMed] [Google Scholar]
- Hanaoka M., Suzuki S., Hotchin J. Thymus-dependent lymphocytes: destruction by lymphocytic choriomeningitis virus. Science. 1969 Mar 14;163(3872):1216–1219. doi: 10.1126/science.163.3872.1216. [DOI] [PubMed] [Google Scholar]
- Henson D., Strano A. J. Mouse cytomegalovirus. Necrosis of infected and morphologically normal submaxillary gland acinar cells during termination of chronic infection. Am J Pathol. 1972 Jul;68(1):183–202. [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Miller J., Najarian J. S. Cytomegalovirus-induced immune suppression. II. Cell-mediated immunity. Clin Exp Immunol. 1974 Sep;18(1):119–126. [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Najarian J. S. Cytomegalovirus-induced immune suppression. I. Humoral immunity. Clin Exp Immunol. 1974 Sep;18(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Joel D. D., Hess M. W., Cottier H. Magnitude and pattern of thymic lymphocyte migration in neonatal mice. J Exp Med. 1972 Apr 1;135(4):907–923. doi: 10.1084/jem.135.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. P. Mouse cytomegalovirus: placental infection. J Infect Dis. 1969 Oct;120(4):445–450. doi: 10.1093/infdis/120.4.445. [DOI] [PubMed] [Google Scholar]
- Jutila J. W. Wasting disease induced with cortisol acetate. 3. Immunologic studies. J Immunol. 1969 Apr;102(4):963–969. [PubMed] [Google Scholar]
- MEDEARIS D. N., Jr MOUSE CYTOMEGALOVIRUS INFECTION. 3. ATTEMPTS TO PRODUCE INTRAUTERINE INFECTIONS. Am J Hyg. 1964 Jul;80:113–120. [PubMed] [Google Scholar]
- MILLER J. F., HOWARD J. G. SOME SIMILARITIES BETWEEN THE NEONATAL THYMECTOMY SYNDROME AND GRAFT-VERSUS-HOST DISEASE. J Reticuloendothel Soc. 1964 Dec;1:369–392. [PubMed] [Google Scholar]
- Mandel T. Differentiation of epithelial cells in the mouse thymus. Z Zellforsch Mikrosk Anat. 1970;106(4):498–515. doi: 10.1007/BF00340288. [DOI] [PubMed] [Google Scholar]
- McCullough B., Krakowka S., Koestner A. Experimental canine distemper virus-induced lymphoid depletion. Am J Pathol. 1974 Jan;74(1):155–170. [PMC free article] [PubMed] [Google Scholar]
- Moore M. A., Owen J. J. Experimental studies on the development of the thymus. J Exp Med. 1967 Oct 1;126(4):715–726. doi: 10.1084/jem.126.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeye R. L. Cytomegalic inclusion disease. The fetal disorder. Am J Clin Pathol. 1967 Jun;47(6):738–744. doi: 10.1093/ajcp/47.6.738. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Shahidi N. T. Thrombocytopenia in murine cytomegalovirus infection. J Lab Clin Med. 1973 Jan;81(1):53–63. [PubMed] [Google Scholar]
- Owen J. J., Ritter M. A. Tissue interaction in the development of thymus lymphocytes. J Exp Med. 1969 Feb 1;129(2):431–442. doi: 10.1084/jem.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER G., BENYESH-MELNICK M. A PLAQUE REDUCTION NEUTRALIZATION TEST FOR HUMAN CYTOMEGALOVIRUS. Proc Soc Exp Biol Med. 1964 Oct;117:145–150. doi: 10.3181/00379727-117-29520. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Vernon M. L., Cross S. S. Classification of mouse thymic virus as a herpesvirus. Infect Immun. 1973 Feb;7(2):305–308. doi: 10.1128/iai.7.2.305-308.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Sorkin E. Hormones and immunologic capacity. I. Effect of heterologous anti-growth hormone (ASTH) antiserum on thymus and peripheral lymphatic tissue in mice. Induction of a wasting syndrome. J Immunol. 1968 Nov;101(5):1036–1043. [PubMed] [Google Scholar]
- RUEBNER B. H., MIYAI K., SLUSSER R. J., WEDEMEYER P., MEDEARIS D. N., Jr MOUSE CYTOMEGALOVIRUS INFECTION. AN ELECTRON MICROSCOPIC STUDY OF HEPATIC PARENCHYMAL CELLS. Am J Pathol. 1964 May;44:799–821. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. N., Daniels C. A., Shivers J. C., Klintworth G. K. Experimental cytomegalovirus ophthalmitis. Am J Pathol. 1974 Dec;77(3):477–492. [PMC free article] [PubMed] [Google Scholar]
- WILSON R., SJODIN K., BEALMEAR M. THE ABSENCE OF WASTING IN THYMECTOMIZED GERMFREE (AXENIC) MICE. Proc Soc Exp Biol Med. 1964 Oct;117:237–239. doi: 10.3181/00379727-117-29545. [DOI] [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F., Toy S. T. Participation of lymphocytes in viral infections. Adv Immunol. 1973;16:123–184. doi: 10.1016/s0065-2776(08)60297-7. [DOI] [PubMed] [Google Scholar]
- White R. G., Boyd J. F. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973 Mar;13(3):343–357. [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H. Immunological responses of 'nude' mice. Clin Exp Immunol. 1971 Feb;8(2):305–317. [PMC free article] [PubMed] [Google Scholar]
- YOUNGNER J. S. Monolayer tissue cultures. I. Preparation and standardization of suspensions of trypsin-dispersed monkey kidney cells. Proc Soc Exp Biol Med. 1954 Feb;85(2):202–205. doi: 10.3181/00379727-85-20830. [DOI] [PubMed] [Google Scholar]