Abstract
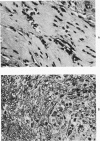
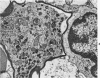
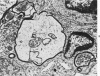
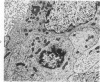
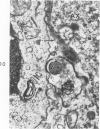
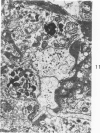
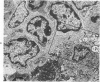
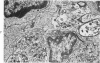
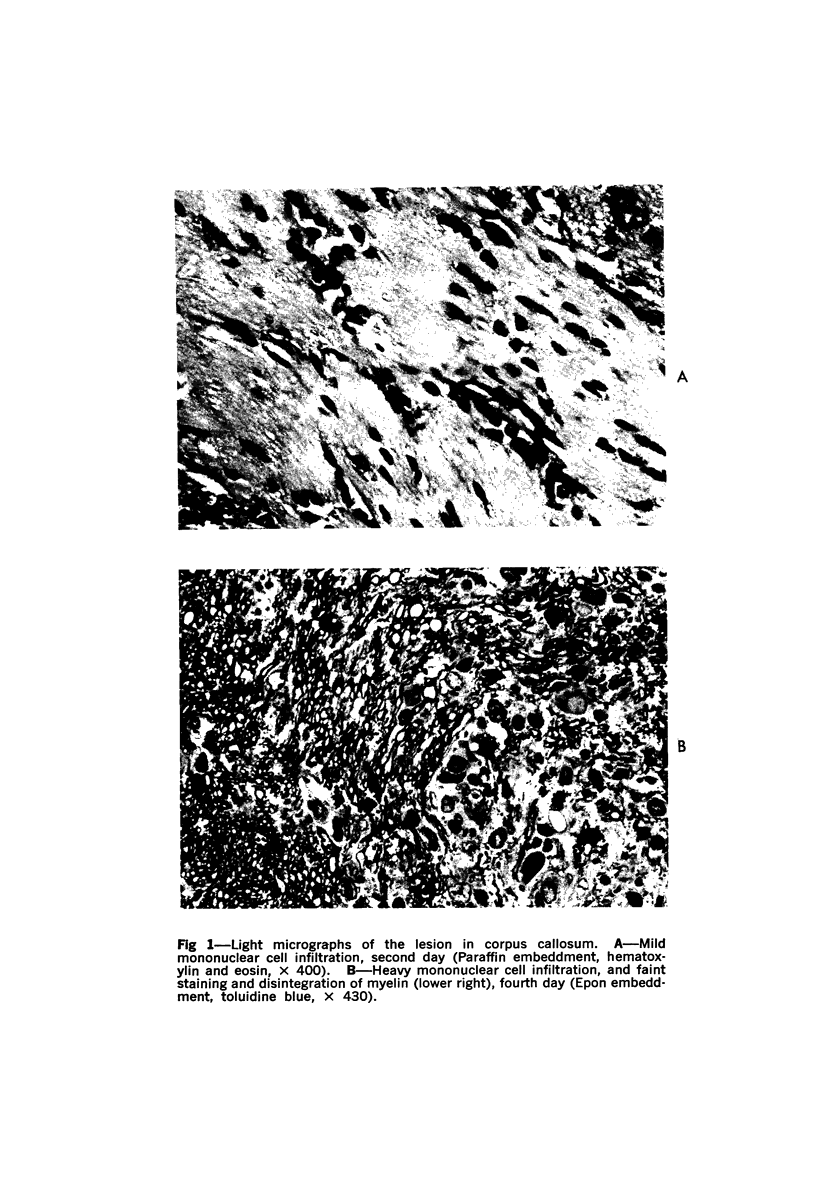
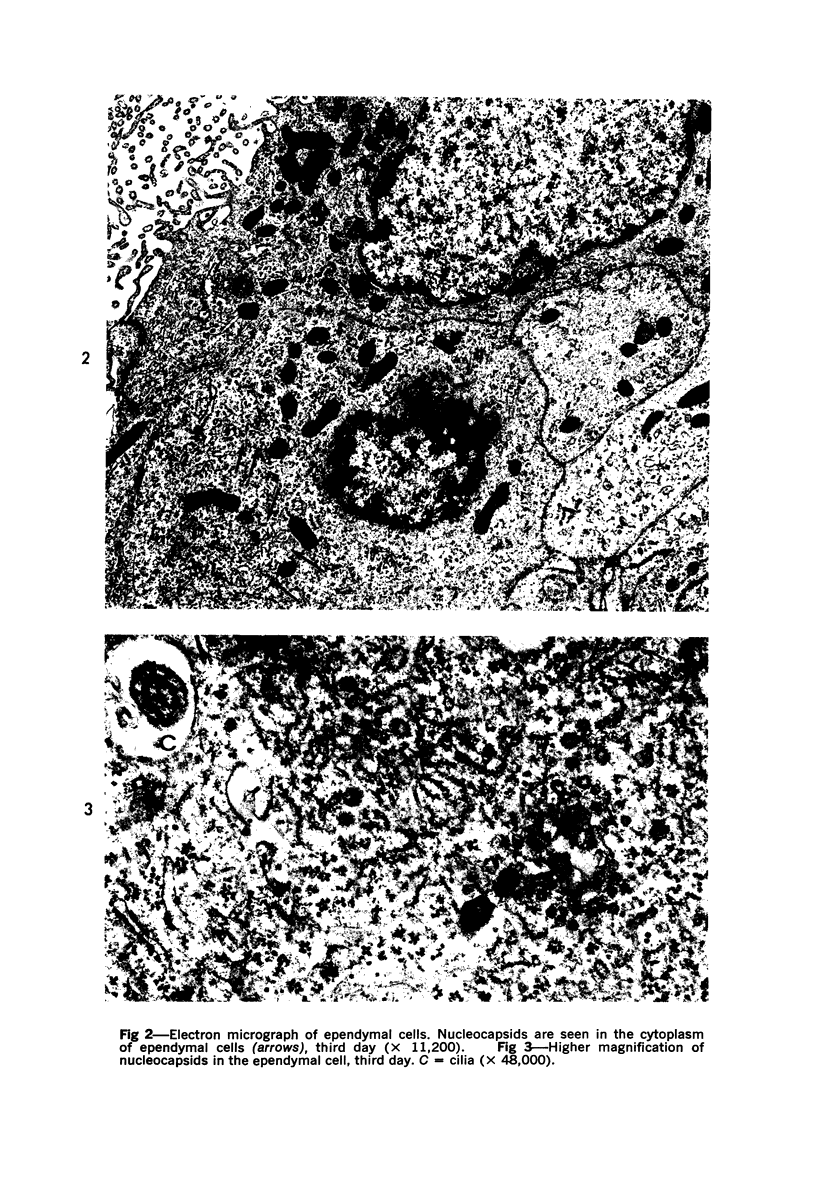
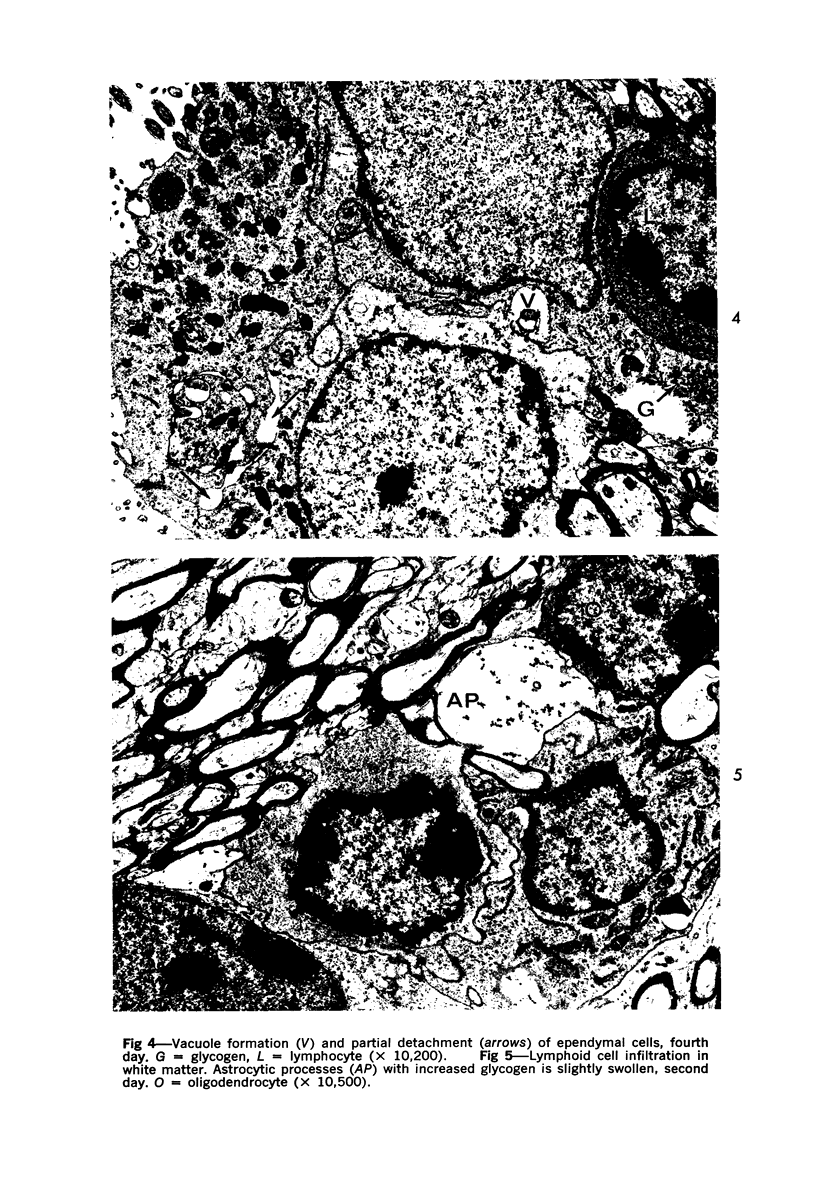
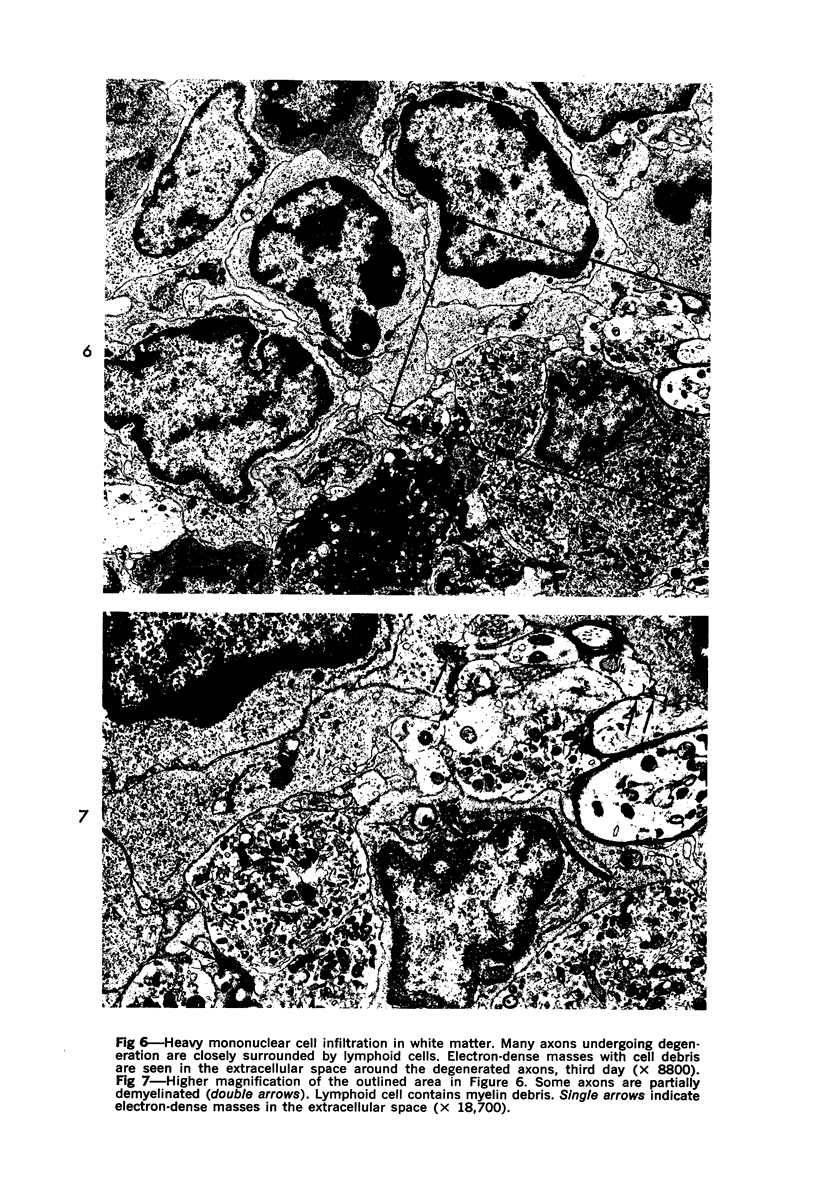
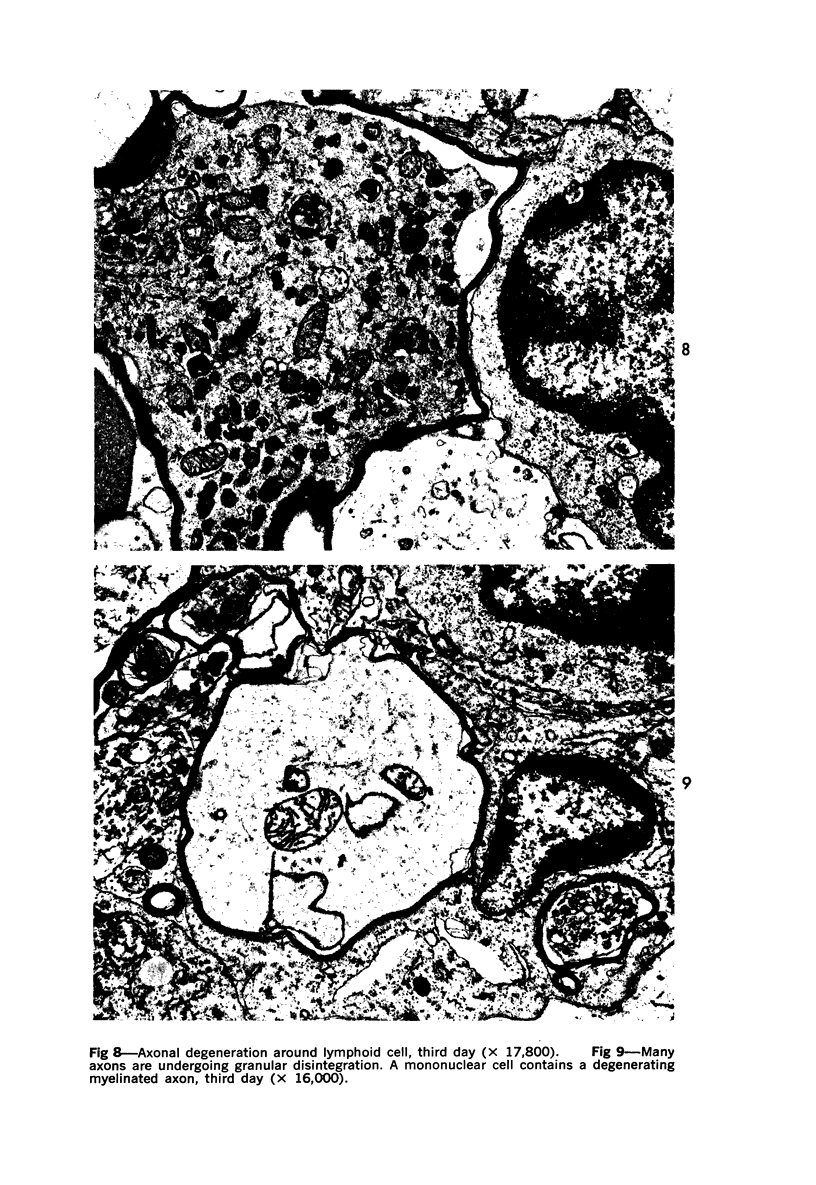
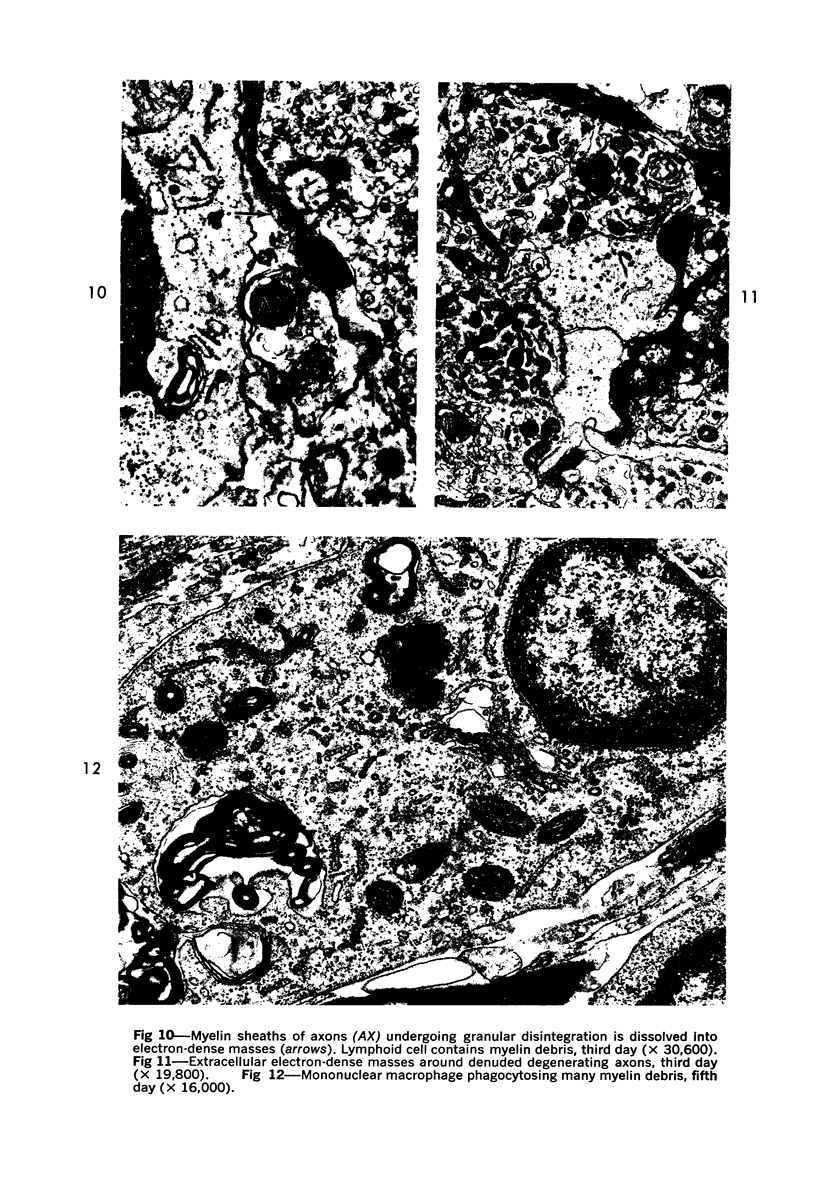
The adult mouse inoculated intracerebrally with 6/94 strain of parainfluenza type 1 virus developed selective degenerative lesions in cerebral white matter. Ultrastrucrally, the infiltration of mononuclear cells, mostly lymphoid cells, apparently preceded the alterations of white matter parenchyma. The prominent feature of the white matter lesion was a lytic degeneration of both axon and myelin that seemed to be triggered by the mononuclear cell infiltration. Nucleocapsids of paramyxovirus were found only in ependymal cells and the very early stages of the infection. It is suggested that the mechanism of the white matter degeneration might be that of a virus-induced cell-mediated immune response directed at both the axon and myelin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld P. Morphogenesis in blood lymphocytes stimulated with phytohaemagglutinin (PHA). A light and electron microscopic study. Acta Pathol Microbiol Scand Suppl. 1971;223(Suppl):1–70. [PubMed] [Google Scholar]
- Byington D. P., Johnson K. P. Experimental subacute sclerosing panencephalitis in the hamster: correlation of age with chronic inclusion-cell encephalitis. J Infect Dis. 1972 Jul;126(1):18–26. doi: 10.1093/infdis/126.1.18. [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Johnson R. T. Granular ependymitis. Occurrence in myxovirus infected rodents and prevalence in man. Am J Pathol. 1972 Jun;67(3):511–526. [PMC free article] [PubMed] [Google Scholar]
- Lampert P. Electron microscopic studies on ordinary and hyperacute experimental allergic encephalomyelitis. Acta Neuropathol. 1967 Oct 20;9(2):99–126. doi: 10.1007/BF00691436. [DOI] [PubMed] [Google Scholar]
- Leduc E. H., Avrameas S., Bouteille M. Ultrastructural localization of antibody in differentiating plasma cells. J Exp Med. 1968 Jan 1;127(1):109–118. doi: 10.1084/jem.127.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. THE FINE STRUCTURE OF THE LYMPHOID TISSUE DURING ANTIBODY FORMATION. Exp Mol Pathol. 1965 Apr;28:155–188. doi: 10.1016/0014-4800(65)90031-6. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Murphy F. A. Parainfluenza virus Sendai infection in macrophages, ependyma, choroid plexus, vascular endothelium and respiratory tract of mice. Am J Pathol. 1973 Mar;70(3):315–328. [PMC free article] [PubMed] [Google Scholar]
- Prineas J. W., Wright R. G. The fine structure of peripheral nerve lesions in a virus-induced demyelinating disease in fowl (Marek's disease). Lab Invest. 1972 May;26(5):548–557. [PubMed] [Google Scholar]
- WAKSMAN B. H., ADAMS R. D. Infectious leukoencephalitis. A critical comparison of certain experimental and naturally-occurring viral leukoencephalitides with experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1962 Oct;21:491–518. [PubMed] [Google Scholar]
- Wiśniewski H., Prineas J., Raine C. S. An ultrastructural study of experimental demyelination and remyelination. I. Acute experimental allergic encephalomyelitis in the peripheral nervous system. Lab Invest. 1969 Aug;21(2):105–118. [PubMed] [Google Scholar]
- Wiśniewski H., Raine C. S., Kay W. J. Observations on viral demyelinating encephalomyelitis. Canine distemper. Lab Invest. 1972 May;26(5):589–599. [PubMed] [Google Scholar]