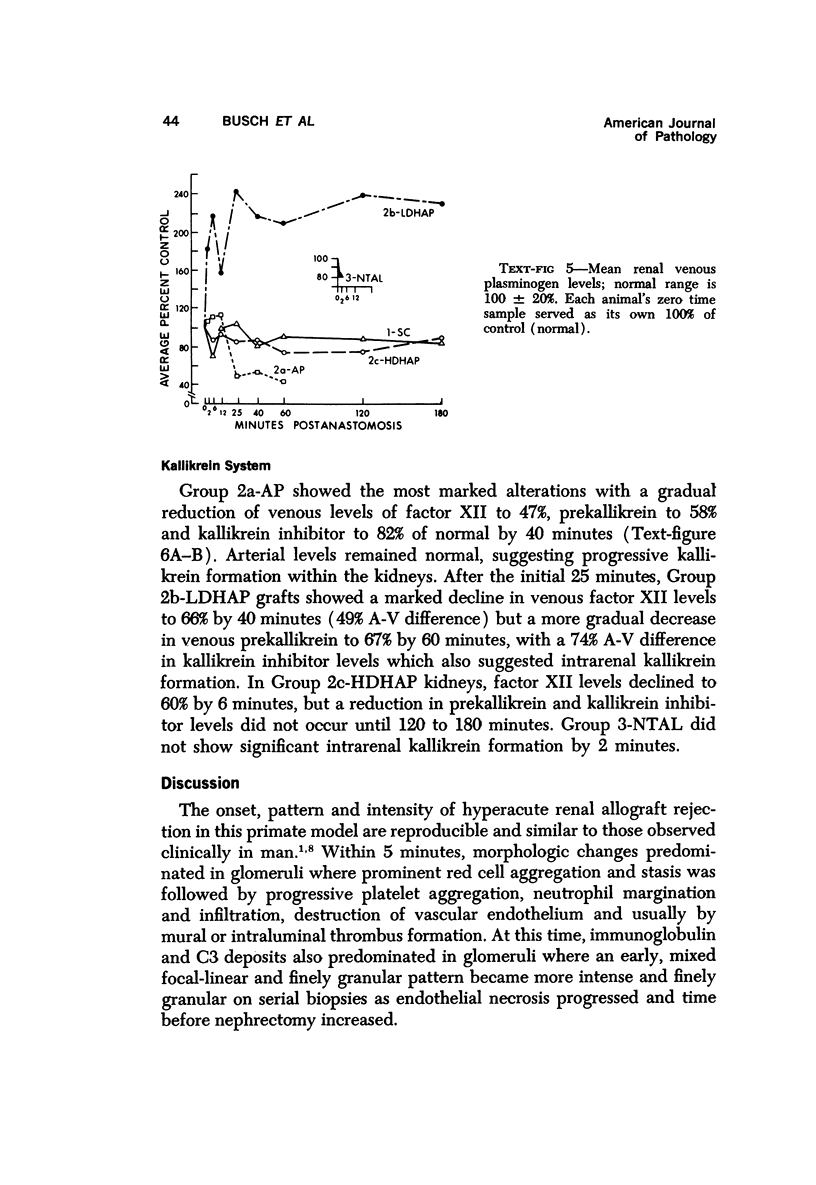
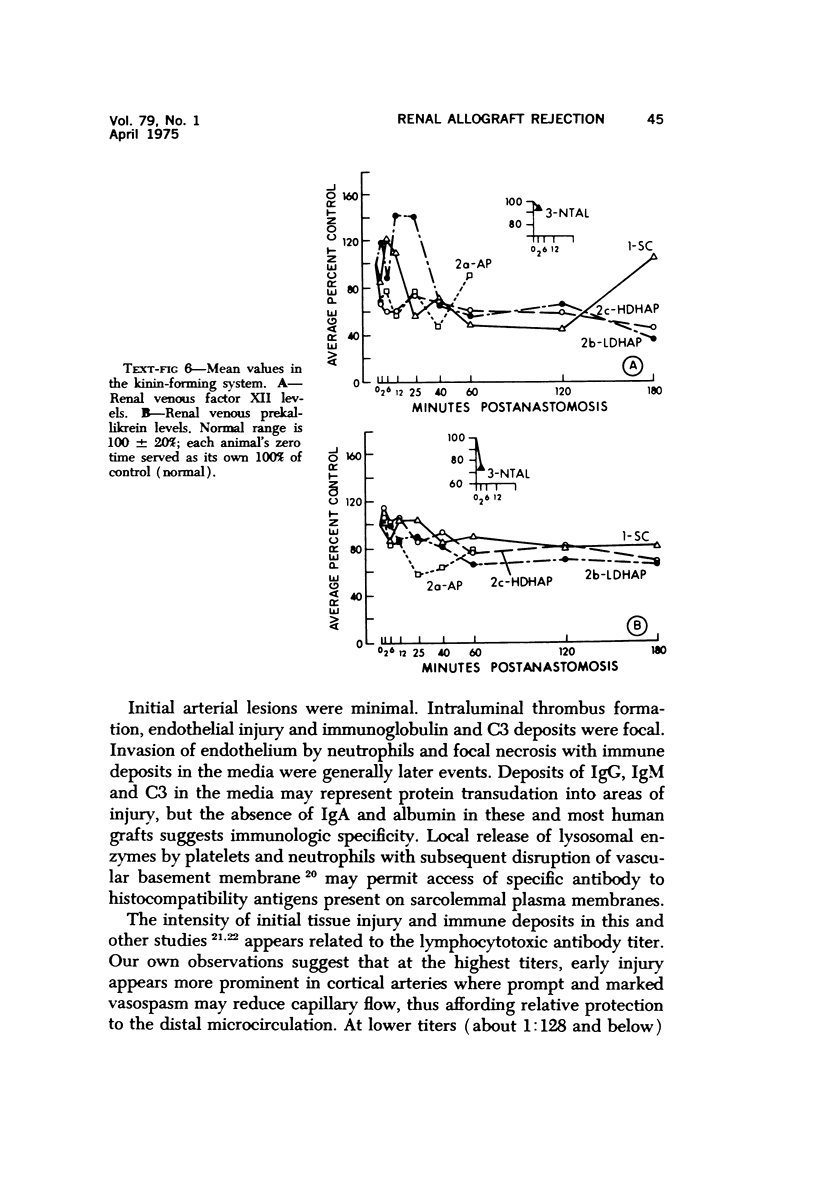
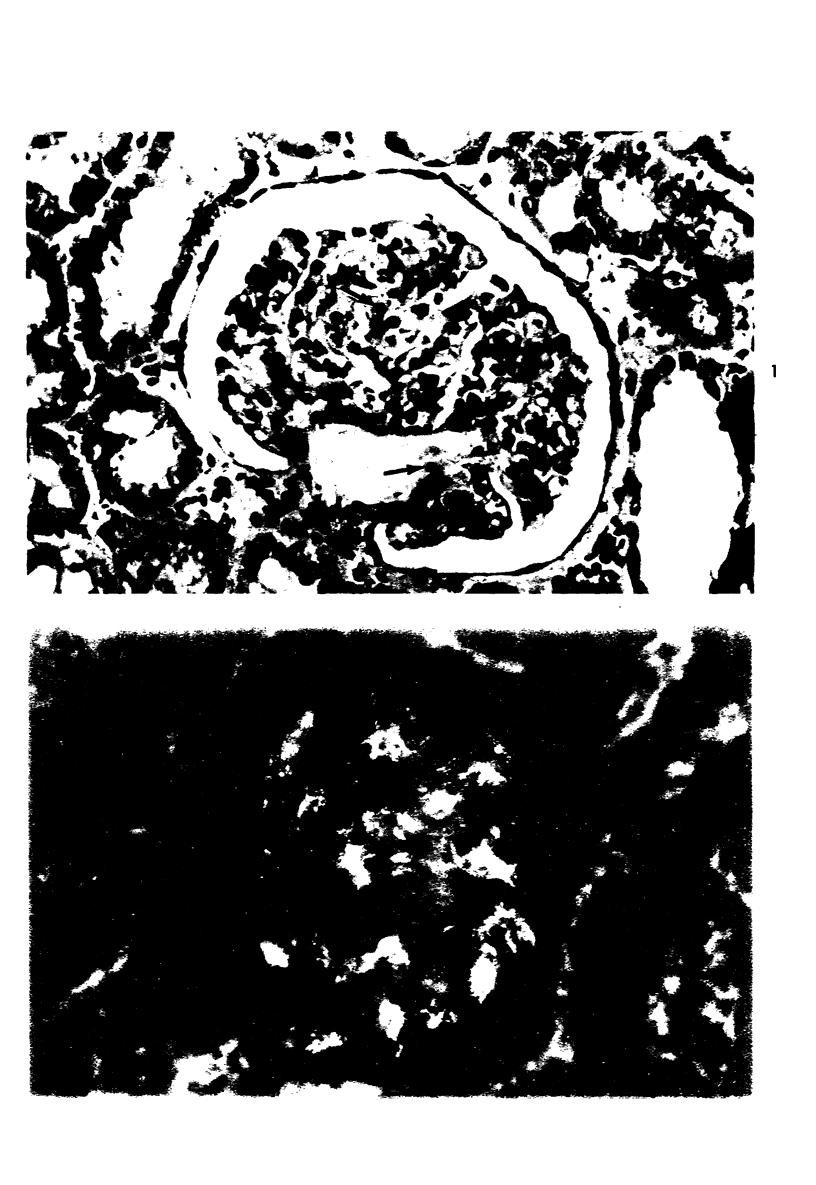
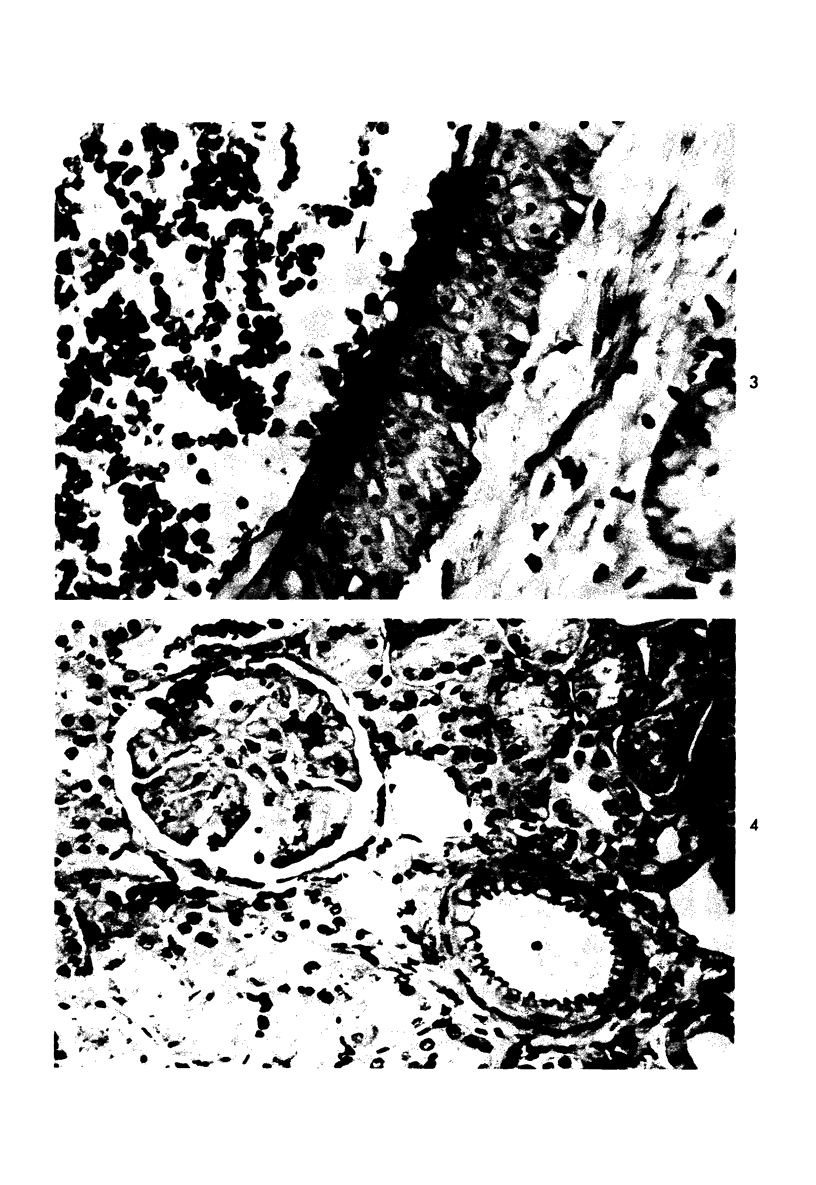
Abstract
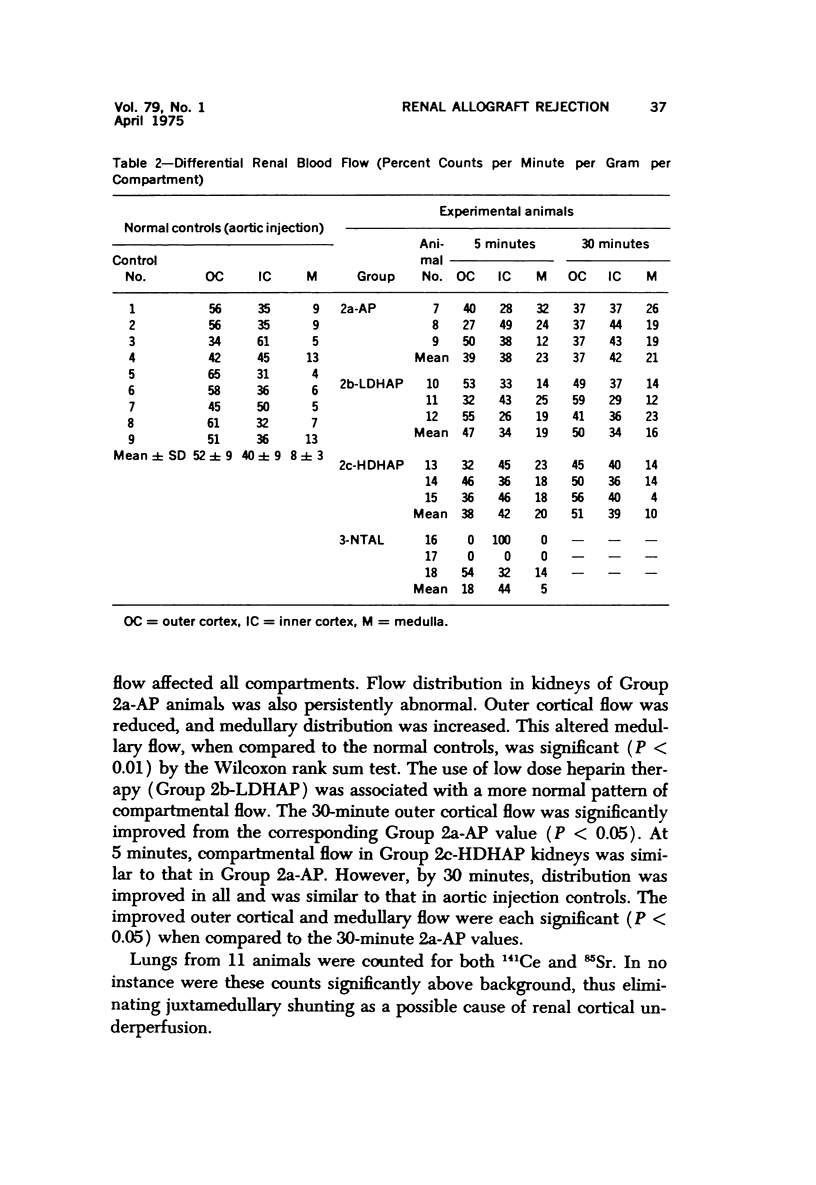
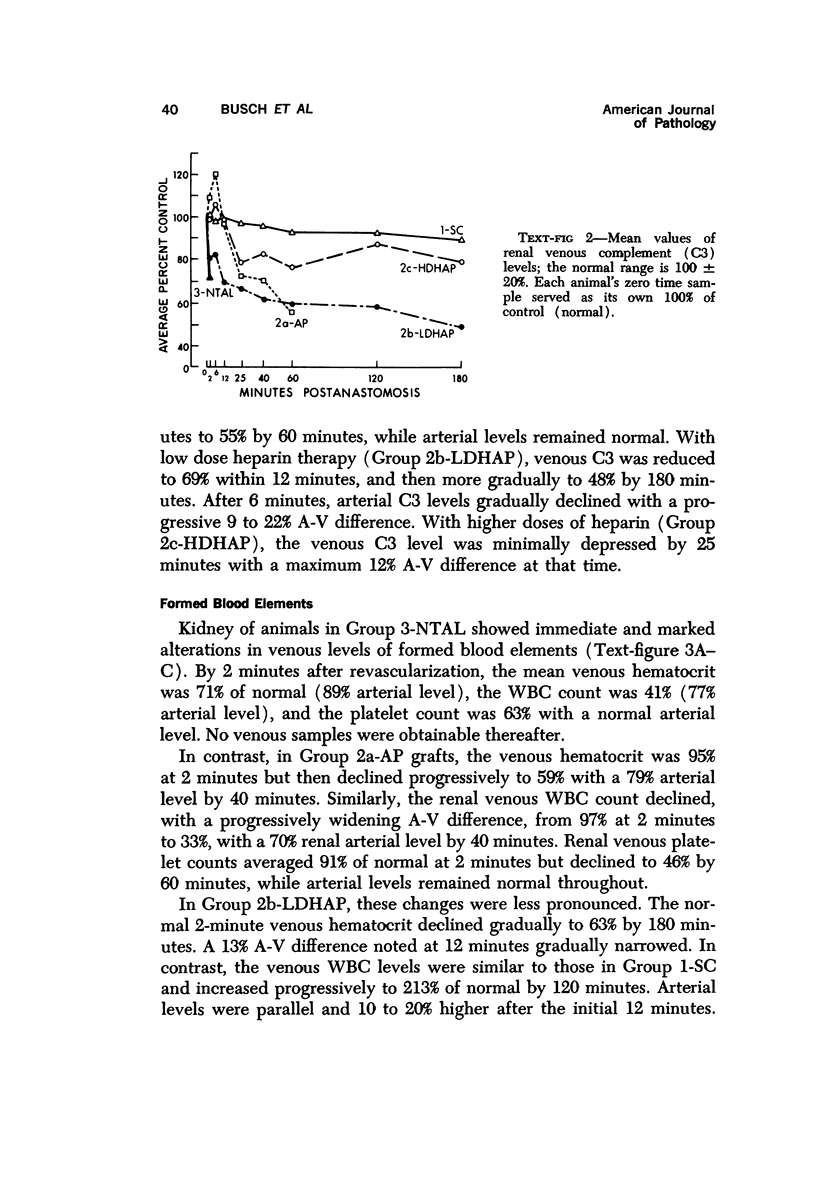
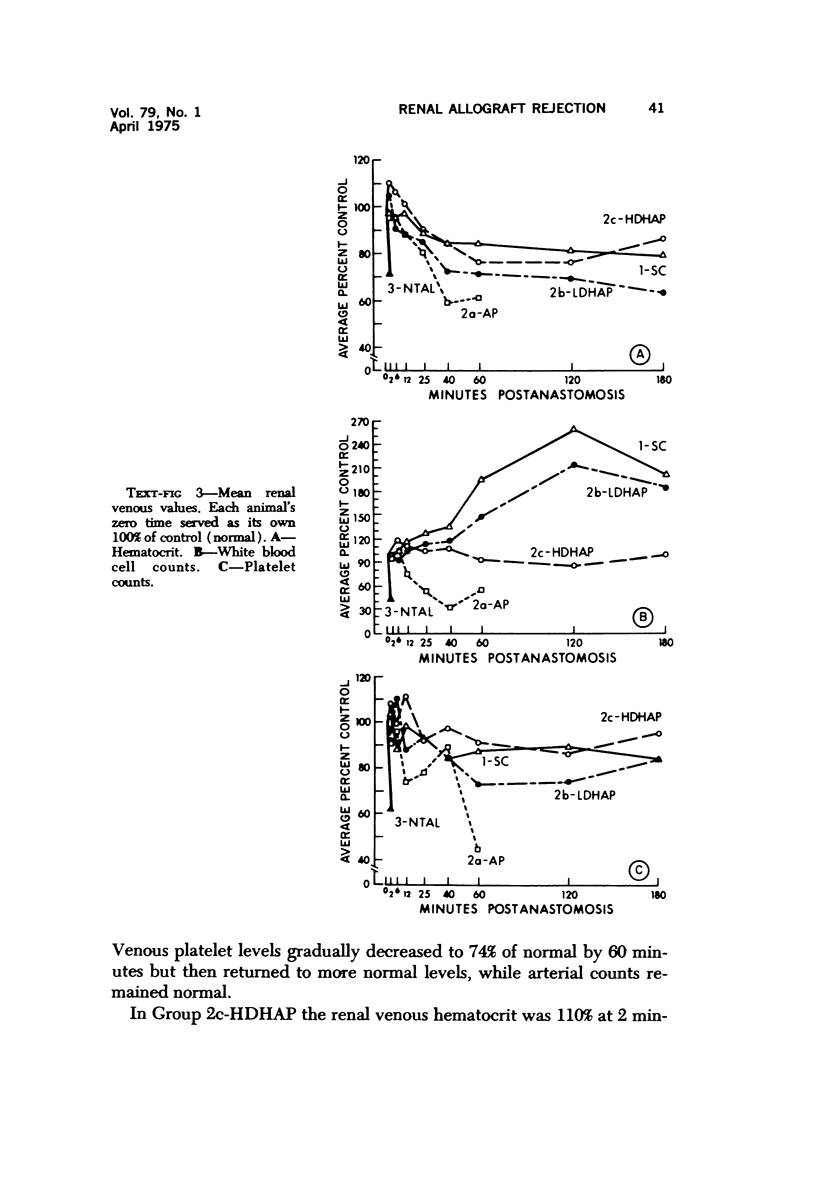
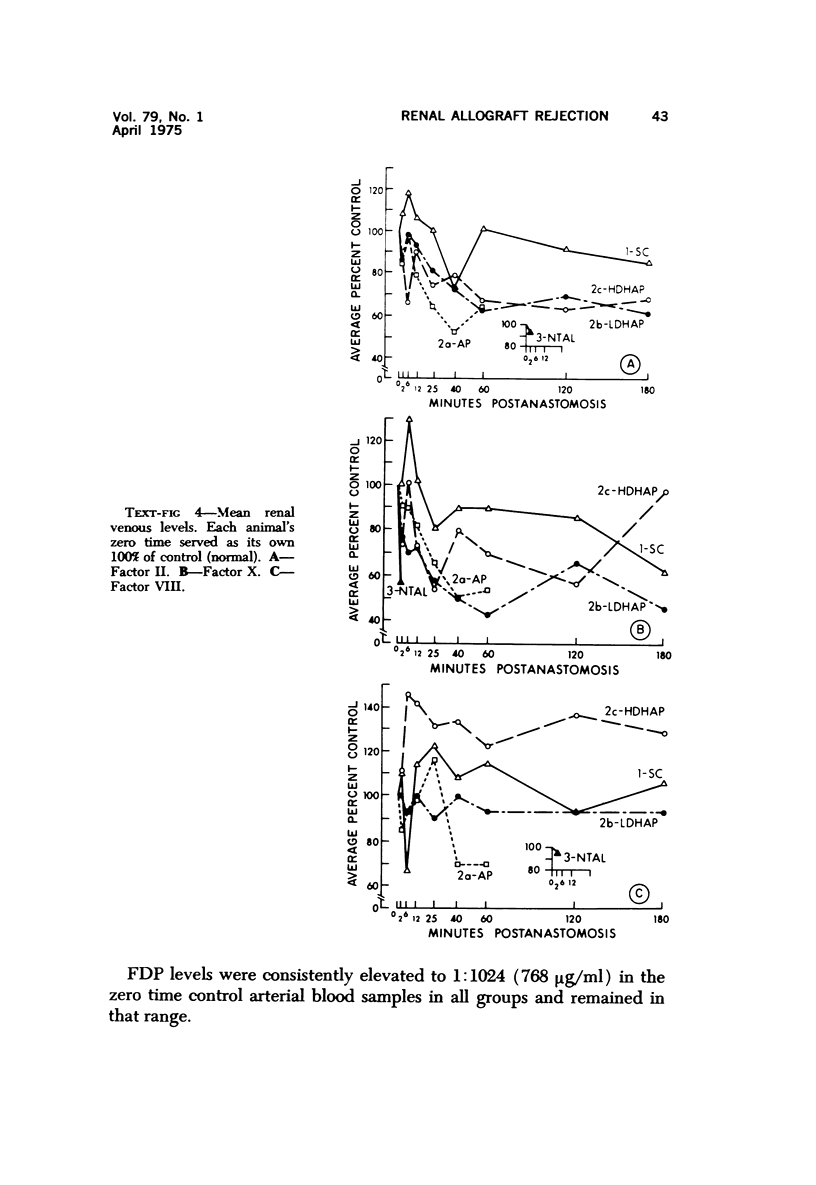
Hyperacute renal allograft rejection is initiated by primary immune injury to vascular endothelium and is propagated by secondary vasoconstriction, platelet aggregation and intravascular coagulation. Previous dissociation of these primary and secondary events, with graft survival in one human, suggested that the more usual graft failure was due to secondary injury. As a basis for further modification studies, this primate model most closely resembled its counterpart in man, as the onset and intensity of functional, morphologic and biochemical alterations were similar. Unmodified allografts failed within 5 minutes. The earliest and most abnormal finding was marked reduction in renal blood flow affecting all compartments. By 5 minutes, histologic changes of hyperacute rejection as well as antibody and faint C3 deposits were noted, but biopsies suggested that the initial flow reduction was more likely due to vasoconstriction, which was then followed by vascular obstruction. Glomeruli appeared most damaged, but at the highest antibody titer arterial injury was more prominent. Early red cell sequestration and stasis was marked, followed by progressive platelet clumping and neutrophil infiltration. While the decline in renal venous C3 levels was prompt, as in man, early intrarenal activation of the coagulation, fibrinolytic and kinin-forming systems could not be demonstrated, and fibrin formation was sparse by light and fluorescence microscopy. Qualitatively similar histologic and functional alterations were noted in autograft controls. While the initiating event was unclear and may have been accentuated by the arteriovenous shunts utilized, the final mechanism was probably marked vasoconstriction with renal ischemia. Intrarenal C3 consumption was an important finding and was not associated with tissue deposits of antibody or complement; it may provide a parallel with the progressive complement-mediated injury associated with acute myocardial ischemia noted by others. Endothelial injury was not seen in arteries, and all alterations were delayed in onset and progressed more slowly than in allografts. These findings may elucidate the mechanism of early malfunction of most autografts. Treatment of additional autografts with increasing doses of heparin progressively reversed these changes and even prevented the initial reduction in blood flow. Therefore, many alterations consistent with hyperacute rejection which are probably immediately responsible for graft failure can also be initiated by nonspecific, nonimmunologic events and, where injury is less intense, can be prevented pharmacologically. This model provides a means of dissecting the injurious events and subsequent evaluation of the effectiveness and interaction of various agents on the damaging secondary alterations which occur during hyperacute rejection.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKJAERSIG N., FLETCHER A. P., SHERRY S. xi-Aminocaproic acid: an inhibitor of plasminogen activation. J Biol Chem. 1959 Apr;234(4):832–837. [PubMed] [Google Scholar]
- Busch G. J., Braun W. E., Carpenter C. B., Corson J. M., Galvanek E. R., Reynolds E. S., Merrill J. P., Dammin G. J. Intravascular coagulation (IVC) in human renal allograft rejection. Transplant Proc. 1969 Mar;1(1):267–270. [PubMed] [Google Scholar]
- Busch G. J., Reynolds E. S., Galvanek E. G., Braun W. E., Dammin G. J. Human renal allografts. The role of vascular injury in early graft failure. Medicine (Baltimore) 1971 Jan;50(1):29–83. [PubMed] [Google Scholar]
- Carpenter C. B., Winn H. J. Hyperacute rejection. N Engl J Med. 1969 Jan 2;280(1):47–48. doi: 10.1056/NEJM196901022800116. [DOI] [PubMed] [Google Scholar]
- Colman R. W., Braun W. E., Busch G. J., Dammin G. J., Merrill J. P. Coagulation studies in the hyperacute and other forms of renal-allograft rejection. N Engl J Med. 1969 Sep 25;281(13):685–691. doi: 10.1056/NEJM196909252811301. [DOI] [PubMed] [Google Scholar]
- Davey F. R., Busch G. J. Immunohistochemistry of glomerulonephritis using horseradish peroxidase and fluorescein-labeled antibody: a comparison of two technics. Am J Clin Pathol. 1970 Apr;53(4):531–536. doi: 10.1093/ajcp/53.4.531. [DOI] [PubMed] [Google Scholar]
- Donaldson V. H. Mechanisms of activation of C'1 esterase in hereditary angioneurotic edema plasma in vitro. J Exp Med. 1968 Mar 1;127(3):411–429. doi: 10.1084/jem.127.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubernard J. M., Carpenter C. B., Busch G. J., Diethelm A. G., Murray J. E. Rejection of canine renal allografts by passive transfer of sensitized serum. Surgery. 1968 Oct;64(4):752–760. [PubMed] [Google Scholar]
- Habal M. B., Kobayashi K., Busch G. J., Birtch A. G. Hyperimmune F(ab') 2 antibody: role in canine hyperacute renal allograft rejection. J Surg Res. 1972 Nov;13(5):228–231. doi: 10.1016/0022-4804(72)90068-6. [DOI] [PubMed] [Google Scholar]
- Hawiger J., Niewiarowski S., Gurewich V., Thomas D. P. Measurement of fibrinogen and fibrin degradation products in serum by staphylococcal clumping test. J Lab Clin Med. 1970 Jan;75(1):93–108. [PubMed] [Google Scholar]
- Janoff A., Zeligs J. D. Vascular injury and lysis of basement membrane in vitro by neutral protease of human leukocytes. Science. 1968 Aug 16;161(3842):702–704. doi: 10.1126/science.161.3842.702. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Hricko G. M., Habal M. B., Lukl P., Busch G. J., Hunsicker L., Reisner G. S., Birtch A. G. Hyperacute rejection of renal allografts in the primate: protective effect of F(ab') 2 fragment of hyperimmune serum. Transplantation. 1972 Sep;14(3):374–381. doi: 10.1097/00007890-197209000-00015. [DOI] [PubMed] [Google Scholar]
- Lucas Z. J., Coplon N., Kempson R., Cohn R. Early renal transplant failure associated with subliminal sensitization. Transplantation. 1970 Dec;10(6):522–529. doi: 10.1097/00007890-197012000-00009. [DOI] [PubMed] [Google Scholar]
- Macdonald A., Busch G. J., Alexander J. L., Pheteplace E. A., Menzoian J., Murray J. E. Heparin and aspirin in the treatment of hyperacute rejection of renal allografts in presensitized dogs. Transplantation. 1970 Jan;9(1):1–7. doi: 10.1097/00007890-197001000-00001. [DOI] [PubMed] [Google Scholar]
- McNay J. L., Abe Y. Pressure-dependent heterogeneity of renal cortical blood flow in dogs. Circ Res. 1970 Oct;27(4):571–587. doi: 10.1161/01.res.27.4.571. [DOI] [PubMed] [Google Scholar]
- Mendell P. L., Hollenberg N. K. Cardiac output distribution in the rat: comparison of rubidium and microsphere methods. Am J Physiol. 1971 Dec;221(6):1617–1620. doi: 10.1152/ajplegacy.1971.221.6.1617. [DOI] [PubMed] [Google Scholar]
- Mimran A., Guiod L., Hollenberg N. K. The role of angiotensin in the cardiovascular and renal response to salt restriction. Kidney Int. 1974 May;5(5):348–355. doi: 10.1038/ki.1974.50. [DOI] [PubMed] [Google Scholar]
- Mittal K. K., Mickey M. R., Singal D. P., Terasaki P. I. Serotyping for homotransplantation. 18. Refinement of microdroplet lymphocyte cytotoxicity test. Transplantation. 1968 Nov;6(8):913–927. doi: 10.1097/00007890-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Patel R., Wilson R. E., Birtch A. G., Merrill J. P., Murray J. E., Busch G. Cadaveric kidney transplantation in a patient with donor-specific antileukocyte cytotoxic antibodies. Report of a case. Transplantation. 1971 Jul;12(1):88–91. doi: 10.1097/00007890-197107000-00017. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. C., Hawkins E., Rector F. Mechanisms of immunological injury during antibody-mediated hyperacute rejection of renal heterografts. Transplantation. 1971 Feb;11(2):151–157. doi: 10.1097/00007890-197102000-00008. [DOI] [PubMed] [Google Scholar]
- Shaipanich T., Vanwijck R. R., Kim J. P., Lukl P., Busch G. J., Wilson R. E. Enhancement of rat renal allografts with F(ab')2 fragment of donor-specific antikidney serum. Surgery. 1971 Jul;70(1):113–121. [PubMed] [Google Scholar]
- THORBURN G. D., KOPALD H. H., HERD J. A., HOLLENBERG M., O'MORCHOE C. C., BARGER A. C. INTRARENAL DISTRIBUTION OF NUTRIENT BLOOD FLOW DETERMINED WITH KRYPTON 85 IN THE UNANESTHETIZED DOG. Circ Res. 1963 Oct;13:290–307. doi: 10.1161/01.res.13.4.290. [DOI] [PubMed] [Google Scholar]
- Wiener A. S., Moor-Jankowski J., Socha W. W. Principles of blood grouping in apes and monkeys: human, simian, and cross-immune types. Transplant Proc. 1972 Mar;4(1):101–105. [PubMed] [Google Scholar]