Abstract
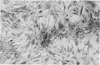
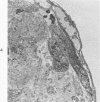
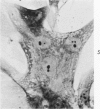
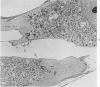
Cells derived from human atherosclerotic plaques and from arterial media were compared with cells obtained from human leiomyomata and myometrium with respect to growth behavior in long-term cell culture. None of numerous variations in culture media, including alterations of serum concentration and source, improved the rate of cell multiplication or in vitro longevity. Both uterine cell types, but neither arterial cell type, multiplied after tissue dissociation with enzymes (elastase, collagenase, hyaluronidase). The replicative life-span of each of eight samples of arterial plaque cells was equal to or less than that of the corresponding medial cells. A similar relationship was observed for eight paired sets of leiomyoma and myometrial cells. The results indicate that, under the conditions of culture in vitro, cells of a bona fide smooth muscle tumor have a finite replicative life-span and smooth muscle cells of atherosclerotic plaques behave in a similar manner.
Full text
PDF

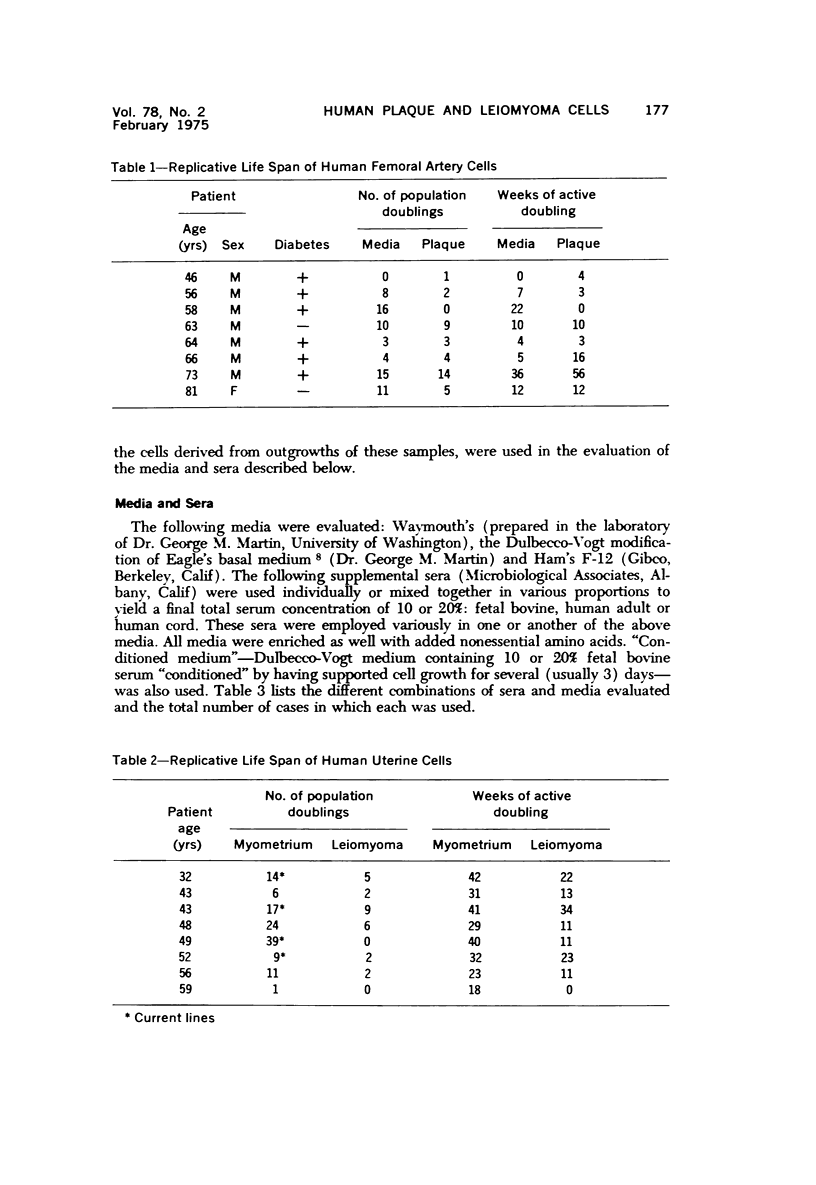
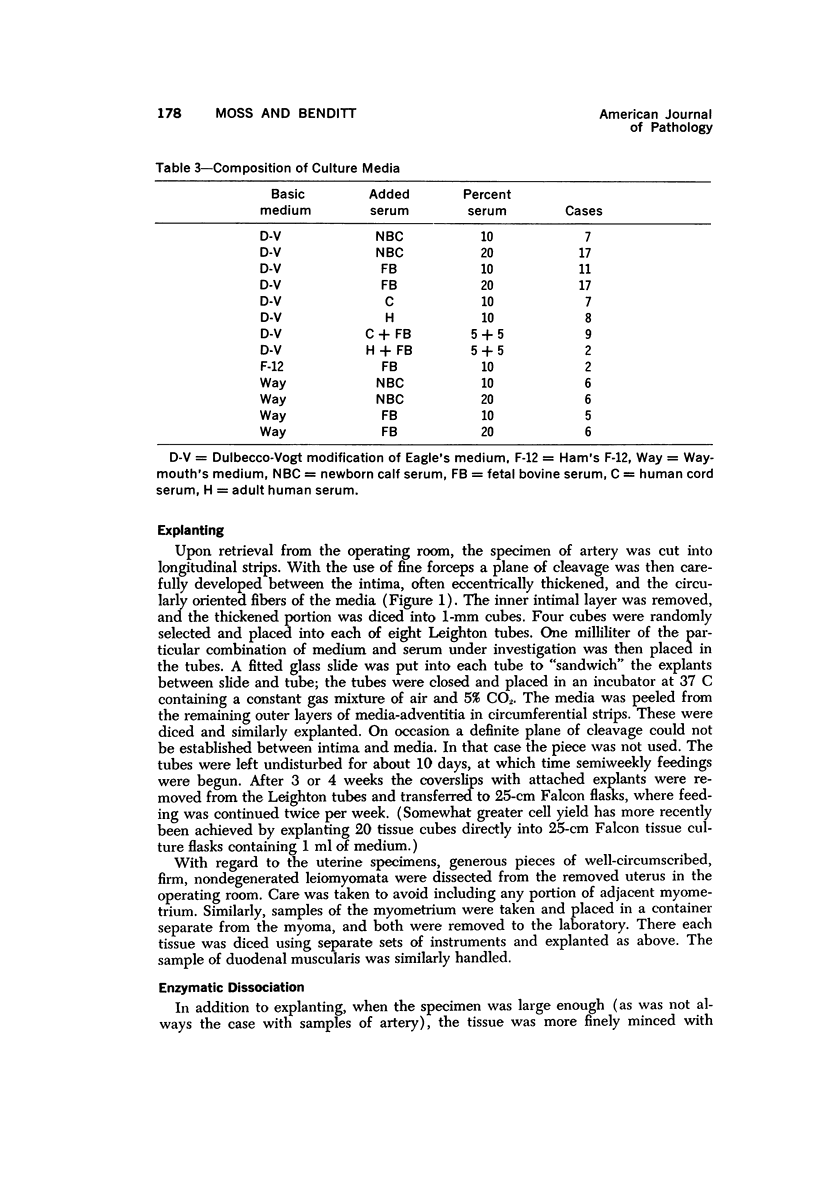








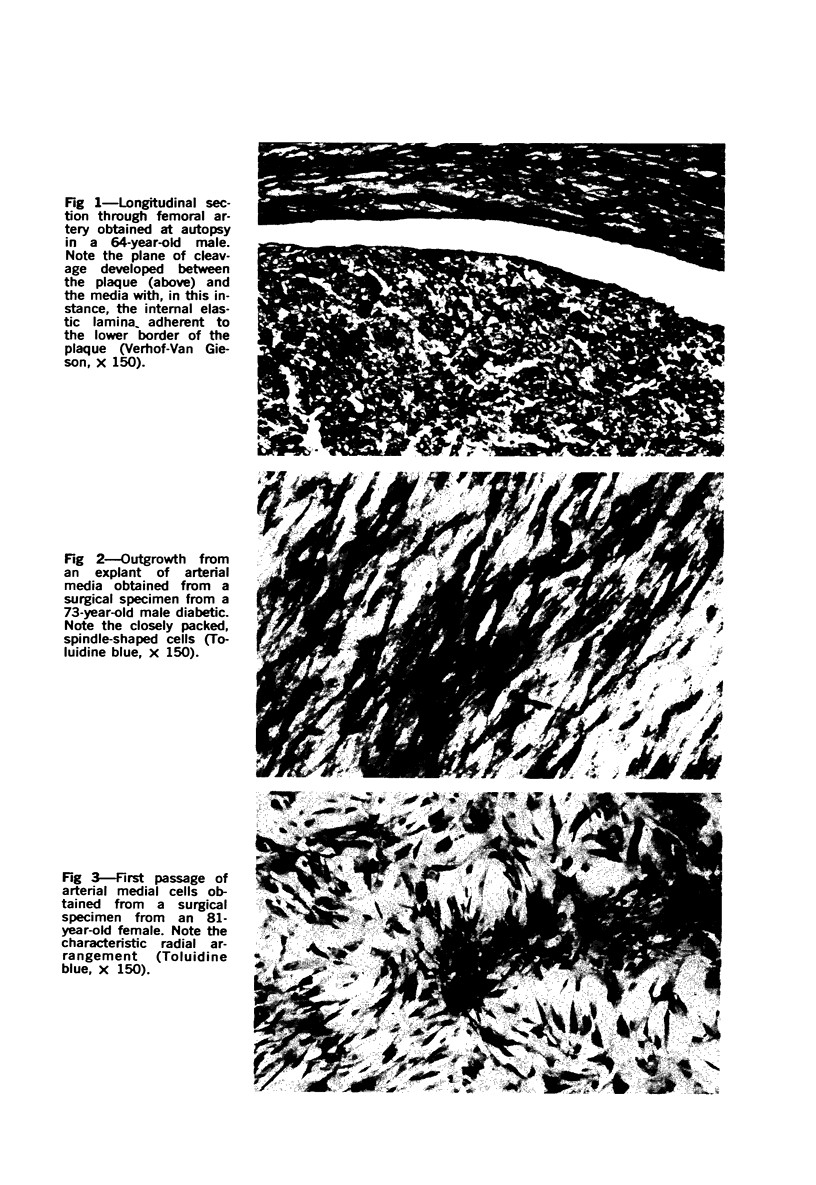


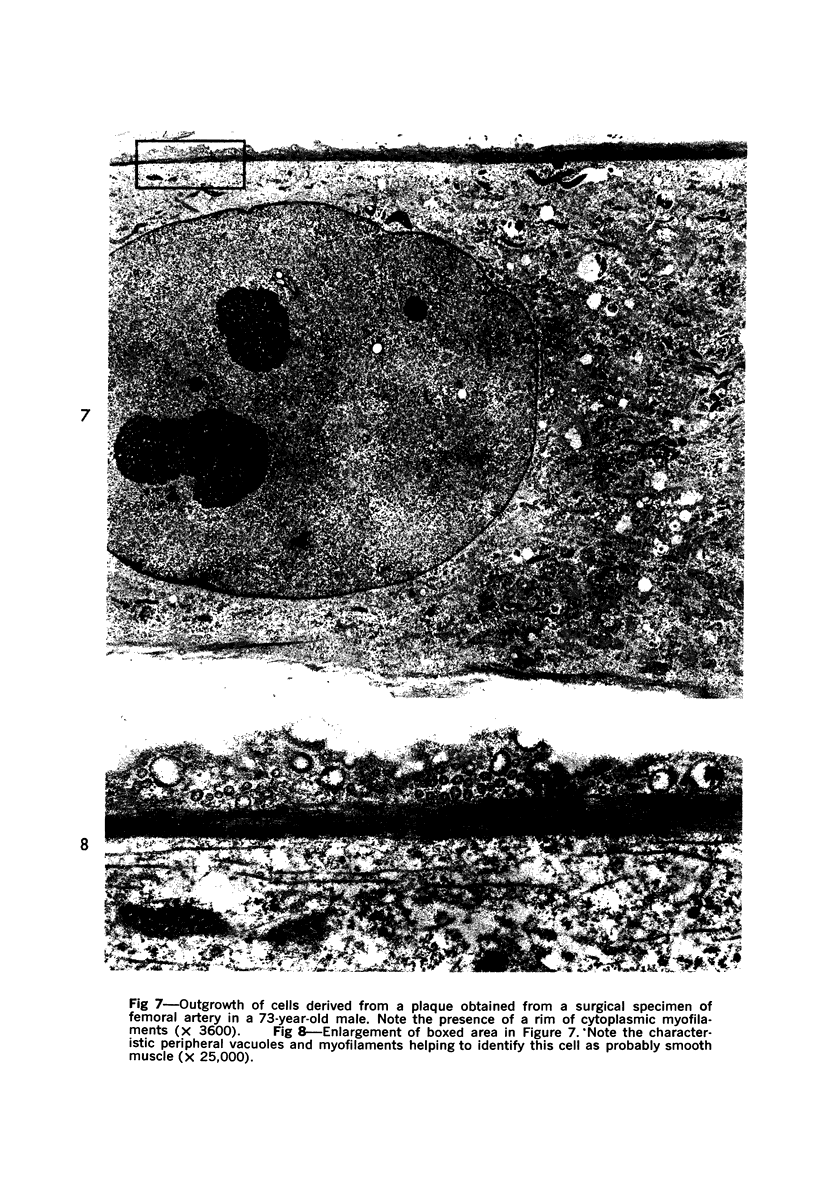
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAOUD A., JARMOLYCH J., ZUMBO A., FANI, FLORENTIN R. PREATHEROMA PHASE OF CORONARY ATHEROSCLEROSIS IN MAN. Exp Mol Pathol. 1964 Oct;90:475–484. doi: 10.1016/0014-4800(64)90028-0. [DOI] [PubMed] [Google Scholar]
- GEER J. C., McGILL H. C., Jr, STRONG J. P. The fine structure of human atherosclerotic lesions. Am J Pathol. 1961 Mar;38:263–287. [PMC free article] [PubMed] [Google Scholar]
- GRAND C. G. Cytologic-tissue culture studies on cervical epithelium. Ann N Y Acad Sci. 1956 Mar 30;63(6):1436–1440. doi: 10.1111/j.1749-6632.1956.tb32148.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Lagunoff D. The in vitro differentiation of mast cells. Cultures of cells from immunized mouse lymph nodes and thoracic duct lymph on fibroblast monolayers. J Cell Biol. 1967 Dec;35(3):685–697. doi: 10.1083/jcb.35.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUST M. D., MORE R. H., MOVAT H. Z. The role of smooth muscle cells in the fibrogenesis of arteriosclerosis. Am J Pathol. 1960 Oct;37:377–389. [PMC free article] [PubMed] [Google Scholar]
- Imai H., Lee K. T., Pastori S., Panlilio E., Florentin R., Thomas W. A. Atherosclerosis in rabbits. Architectural and subcellular alterations of smooth muscle cells of aortas in response to hyperlipemia. Exp Mol Pathol. 1966 Jun;5(3):273–310. doi: 10.1016/0014-4800(66)90036-0. [DOI] [PubMed] [Google Scholar]
- KALTENBACH J. P., KALTENBACH M. H., LYONS W. B. Nigrosin as a dye for differentiating live and dead ascites cells. Exp Cell Res. 1958 Aug;15(1):112–117. doi: 10.1016/0014-4827(58)90067-3. [DOI] [PubMed] [Google Scholar]
- Lipetz J., Cristofalo V. J. Ultrastructural changes accompanying the aging of human diploid cells in culture. J Ultrastruct Res. 1972 Apr;39(1):43–56. doi: 10.1016/s0022-5320(72)80005-4. [DOI] [PubMed] [Google Scholar]
- MOORE J. G. Growth characteristics in tissue culture of controversial lesions of the uterine cervix. West J Surg Obstet Gynecol. 1955 Jan;63(1):1–9. [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. 3. The cholesterol-induced lesions and the effect of a cholesterol and oil diet on the preexisting spontaneous plaque in the chicken aorta. Lab Invest. 1970 Nov;23(5):521–535. [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. II. The spontaneous plaque in the chicken. Lab Invest. 1970 Sep;23(3):231–245. [PubMed] [Google Scholar]
- Robbins E., Levine E. M., Eagle H. Morphologic changes accompanying senescence of cultured human diploid cells. J Exp Med. 1970 Jun 1;131(6):1211–1222. doi: 10.1084/jem.131.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Transformation induced by simian virus 40 in human renal cell cultures. I. Morphology and growth characteristics. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1164–1172. doi: 10.1073/pnas.48.7.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szemenyei K., Kóczé A., Jellinek H. Experimental injury of muscular-type blood vessels by chemical agents. Acta Morphol Acad Sci Hung. 1968;16(2):157–163. [PubMed] [Google Scholar]
- TODARO G. J., NILAUSEN K., GREEN H. GROWTH PROPERTIES OF POLYOMA VIRUS-INDUCED HAMSTER TUMOR CELLS. Cancer Res. 1963 Jul;23:825–832. [PubMed] [Google Scholar]
- TODARO G. J., WOLMAN S. R., GREEN H. RAPID TRANSFORMATION OF HUMAN FIBROBLASTS WITH LOW GROWTH POTENTIAL INTO ESTABLISHED CELL LINES BY SV40. J Cell Physiol. 1963 Dec;62:257–265. doi: 10.1002/jcp.1030620305. [DOI] [PubMed] [Google Scholar]
- Wissler R. W. The arterial medial cell, smooth muscle, or multifunctional mesenchyme? Circulation. 1967 Jul;36(1):1–4. doi: 10.1161/01.cir.36.1.1. [DOI] [PubMed] [Google Scholar]