Abstract
The Streptococcus gordonii cell surface glycoprotein GspB mediates high-affinity binding to distinct sialylated carbohydrate structures on human platelets and salivary proteins. GspB is glycosylated in the cytoplasm of S. gordonii and is then transported to the cell surface via a dedicated transport system that includes the accessory Sec components SecA2 and SecY2. The means by which the GspB preprotein is selectively recognized by the accessory Sec system have not been characterized fully. GspB has a 90-residue amino-terminal signal sequence that displays a traditional tripartite structure, with an atypically long amino-terminal (N) region followed by hydrophobic (H) and cleavage regions. In this report, we investigate the relative importance of the N and H regions of the GspB signal peptide for trafficking of the preprotein. The results show that the extended N region does not prevent export by the canonical Sec system. Instead, three glycine residues in the H region not only are necessary for export via the accessory Sec pathway but also interfere with export via the canonical Sec route. Replacement of the H-region glycine residues with helix-promoting residues led to a decrease in the efficiency of SecA2-dependent transport of the preprotein and a simultaneous increase in SecA2-independent translocation. Thus, the hydrophobic core of the GspB signal sequence is responsible primarily for routing towards the accessory Sec system.
GspB is a cell surface glycoprotein expressed by Streptococcus gordonii that mediates the high-affinity binding of this organism to human platelets (8). This unusual adhesin is a member of an expanding family of serine-rich glycoproteins which includes Fap1 of Streptococcus parasanguinis, Hsa of S. gordonii Challis, SraP of Staphylococcus aureus, the Srr proteins of Streptococcus agalactiae, and the SrpA proteins of Streptococcus sanguinis and Streptococcus cristatus (21, 35, 40, 42, 44, 56). Although identified only relatively recently, in part because of their extremely high apparent molecular mass and failure to react with conventional protein stains, these glycoproteins have been found not only to bind specific receptors on human tissues (7, 35, 45, 47, 48, 57) but also to contribute to virulence, as measured by animal models of infection (32, 40, 42, 46).
Little is known thus far regarding the role of the carbohydrate residues on these glycoproteins. Our studies with GspB have indicated that the carbohydrate moieties are essential for maintaining the stability and solubility of this adhesin (9, 49). Two serine-rich regions of GspB (SRR1 and SRR2) (Fig. 1) become glycosylated through the coordinated activity of four proteins (Gly, Nss, GtfA, and GtfB) encoded downstream of gspB (49, 50). GtfA and GtfB are absolutely essential for glycosylation, whereas Gly and Nss affect the overall carbohydrate composition. The addition of carbohydrate residues takes place in the cytoplasm of S. gordonii and occurs extremely rapidly, possibly concomitant with synthesis of the GspB polypetide.
FIG. 1.
Accessory sec locus of S. gordonii M99 and domain organization of GspB. Upper diagram, map of the 23-kb M99 chromosomal locus containing gspB and the accessory sec genes. Asp1-5, SecY2, and SecA2 are components of the accessory Sec system. Gly, Nss, GtfA, and GtfB affect the glycosylation of GspB. Lower diagram, GspB polypeptide domains. SS, signal sequence; SRR1, first serine-rich region; BR, basic region; SRR2, second serine-rich region; CWA, cell wall-anchoring domain. GspB736flag is a variant of GspB that is truncated at residue 736 of 3,072 residues and has a C-terminal 3XFLAG tag.
The region downstream of gspB also encodes seven proteins (SecA2, SecY2, and Asp1 through Asp5) that comprise an accessory Sec system, which is dedicated to the transport of GspB (8, 50, 51). SecA2 is highly similar to SecA, an ATPase and essential component of the canonical Sec system. SecY2 is highly similar to SecY, whereas Asp4 and Asp5 may interact with SecY2 to form a heterotrimeric protein-conducting channel similar to the SecYEG transmembrane translocase. Asp1, Asp2, and Asp3 do not resemble any proteins of known function but are essential for transport of GspB from the S. gordonii cytoplasm.
The structural features of GspB that specifically target this glycoprotein towards the accessory Sec system are not well defined. Studies using C-terminally truncated variants of GpsB have indicated that the cell wall-anchoring domain (LPXTG motif, hydrophobic domain and charged tail [20, 38, 39]) does not play a role in export (6, 9). These variants provide a useful tool for examining export, since they do not become covalently linked to the cell wall peptidoglycan but rather are freely secreted into the culture medium. As is the case for full-length GspB, the export of these variants is entirely dependent on the accessory Sec system. That is, disruption of secA2, secY2, or any of the asp genes results in a loss of export and the accumulation of the glycosylated variants in the bacterial cytoplasm. We have used a FLAG-tagged variant of GspB that is truncated at residue 736 (GspB736flag) to examine features of GspB that are essential for export via the accessory Sec pathway (9). Unlike longer variants of GspB, the polypeptide backbone of GspB736flag is stable in the absence of glycosylation, which permits the analysis of translocation independently of the carbohydrate modifications. Experiments using this truncated variant revealed that nonglycosylated GspB736flag can be exported by the canonical Sec system, albeit inefficiently. However, the addition of carbohydrate residues to GspB736flag can inhibit export via that pathway. Although these carbohydrate moieties are readily accommodated by the accessory Sec system, they do not direct GspB towards that route. Instead, an N-terminal 90-amino-acid cleavable signal sequence is necessary for export via the accessory Sec pathway.
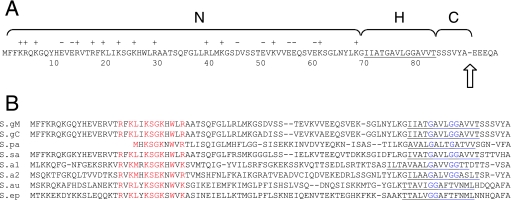
The GspB signal sequence consists of a traditional tripartite structure (54), with a positively charged amino-terminal (N) region, a core hydrophobic (H) region, and a polar cleavage region (Fig. 2A). However, the N region is approximately three times longer than the corresponding region of canonical signal peptides (55), and contains many acidic residues. It was initially supposed that the extended N region might interfere with export via the canonical Sec pathway and could therefore be critical for trafficking. Somewhat unexpectedly, a deletion within the atypically long N region (residues 8 to 68) which was predicted to yield a canonical signal sequence not only impaired export of the glycosylated variant of GspB736flag via the accessory Sec pathway but also abolished export of the nonglycosylated variant via the canonical Sec route. This suggested that other domains of the signal sequence might facilitate the selective targeting of the GspB preprotein. In this study, we looked for conserved features in the signal sequences of GspB and several of its homologues in order to identify domains responsible for trafficking towards the accessory Sec system. The results indicate that glycine residues within the H region of the signal sequence promote export via the accessory Sec pathway and inhibit export via the canonical Sec route.
FIG. 2.
(A) Characteristics of the GspB signal sequence. N, H, and C refer to the amino-terminal, hydrophobic, and cleavage regions, respectively. Basic (+) and acidic (−) amino acid residues in the N region are indicated. Residues comprising the H region are underlined. The arrow indicates the location of an experimentally determined cleavage point. (B) Comparison of the amino-terminal ends of selected GspB homologues. The sequences were aligned with the ClustalW algorithm (www.ebi.ac.uk/clustalw), using the default parameters. S.gM, S. gordonii M99 (GenBank accession number AAL13053); S.gC, S. gordonii Challis (GenBank accession number BAA97453); S.pa, S. parasanguinis (GenBank accession number AAC79868); S.sa, S. sanguinis (obtained from the Virginia Commonwealth University sequencing project at www.sanguinis.mic.vcu.edu); S.a1, S. agalactiae COH1 (GenBank accession number EAO76299); S.a2, S. agalactiae 2603V/R (GenBank accession number AAN00330); S.au, Staphylococcus aureus (GenBank accession number ABD31977); S.ep, Staphylococcus epidermidis (GenBank accession number AAO05891).
MATERIALS AND METHODS
Bacterial strains, plasmids, and reagents.
The bacterial strains and plasmids used in this study are listed in Table 1. S. gordonii strains were grown in Todd-Hewitt broth (THB; Difco Laboratories) at 37°C in a 5% CO2 environment. Dulbecco's phosphate-buffered saline (DPBS), peroxidase-conjugated antibodies, anti-FLAG M2 monoclonal antibody, and anti-FLAG M2 agarose were obtained from Sigma. Biotinylated succinylated wheat germ agglutinin (sWGA) was from Vector Laboratories, and peroxidase-conjugated streptavidin was from Pierce.
TABLE 1.
Strains and plasmids used in the present study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| M99 | Streptococcus gordonii parental strain | 43 |
| PS516 | M99 ΔsecA2; in-frame deletion | 8 |
| PS727 | PS516 ΔgtfA::spc; Specr | This study |
| PS846 | M99 ΔgspB::pEVP3; Cmr | 9 |
| PS919 | M99 gspB::pB736flag; Ermr | 9 |
| PS946 | M99 gspB::pB736flagR; gspB merodiploid; Ermr | 9 |
| PS961 | PS846 gspB::pB736flagR; Cmr Ermr | This study |
| PS998 | PS516 ΔgspB::pEVP3; Cmr | This study |
| PS999 | PS727 ΔgspB::pEVP3; Cmr Specr | This study |
| PS1027 | PS846 gspB::pB736flagRΔ8-68; Cmr Ermr | 9 |
| PS1033 | PS1027 ΔgtfA::spc; Cmr Ermr Specr | 9 |
| PS1115 | PS846 gspB::pB736flagR/A79C80; Cmr Ermr | This study |
| PS1129 | PS846 gspB::pB736flagR/L75A79C80; Cmr Ermr | This study |
| PS1130 | PS1115 ΔgtfA::spc; Cmr Ermr Specr | This study |
| PS1132 | PS998 gspB::pB736flagR/A79C80; Cmr Ermr | This study |
| PS1133 | PS1132 ΔgtfA::spc; Cmr Ermr Specr | This study |
| PS1136 | PS1129 ΔgtfA::spc; Cmr Ermr Specr | This study |
| PS1137 | PS999 gspB::pB736flagR/L75A79C80; Cmr Ermr Specr | This study |
| PS1139 | PS999 gspB::pB736flagRΔ8-68/L75A79C80; Cmr Ermr Specr | This study |
| PS1144 | M99 gspB::pB736flagR L75A79C80; gspB merodiploid; Ermr | This study |
| PS1146 | PS998 gspB::pB736flagR/L75A79C80; Cmr Ermr | This study |
| PS1171 | PS1138 ΔgtfA::spc; Cmr Ermr Specr | This study |
| PS1172 | PS1147 ΔgtfA::spc; Cmr Ermr Specr | This study |
| PS1176 | PS998 gspB::pB736flagR; Cmr Ermr | This study |
| PS1177 | PS999 gspB::pB736flagR; Cmr Ermr | This study |
| PS1179 | PS961 ΔgtfA::spc; Cmr Ermr Specr | This study |
| PS1203 | PS846 gspB::pB736flagR/G75L; Cmr Ermr | This study |
| PS1204 | PS998 gspB::pB736flagR/G75L; Cmr Ermr | This study |
| PS1205 | PS999 gspB::pB736flagR/G75L; Cmr Ermr Specr | This study |
| PS1206 | PS846 gspB::pB736flagR/G75P; Cmr Ermr | This study |
| PS1207 | PS998 gspB::pB736flagR/G75P; Cmr Ermr | This study |
| PS1208 | PS999 gspB::pB736flagR/G75P; Cmr Ermr Specr | This study |
| PS1211 | PS1203 ΔgtfA::spc; Cmr Ermr Specr | This study |
| PS1212 | PS1206 ΔgtfA::spc; Cmr Ermr Specr | This study |
| Plasmids | ||
| pVA891 | erm (gram positive), cat tet ori (E. coli) | 30 |
| pEVP3 | cat (gram positive and E. coli), ori (E. coli) | 15 |
| pB736flag | gspB codons 604 to 736 with a 3XFLAG tag in pVA891 | 9 |
| pB736flagR | gspB736flag and pVA891 rescued from PS919 | 9 |
| pB736flagRΔ8-68 | pB736flagR with deletion of the indicated codons | 9 |
| pB736flagR/G75L | pB736flagR with an alteration of the indicated codon | This study |
| pB736flagR/G75P | pB736flagR with an alteration of the indicated codon | This study |
| pB736flagR/G79A/G80C | pB736flagR with an alteration of the indicated codons | This study |
| pB736flagR*G3 | pB736flagR with codon substitutions G75L G79A G80C | This study |
| pB736flagR*G3Δ8-68 | pB736flagRΔ8-68 with three glycine codon substitutions | This study |
Specr, spectinomycin resistant; Cmr, chloramphenicol resistant; Ermr, erythromycin resistant; Ampr, ampicillin resistant.
Targeted substitutions within the signal peptide region of GspB736flag.
Mutations in the gspB736flag sequence were made by a two-stage PCR procedure. For conversion of codon 75 from glycine to leucine, primers 5′-ACACTTTGATTGGAATGCTCGG-3′ (32F) and 5′-CCACCAAGCACTGCTAGCGTCGCTATAATTCC-3′ or primers 5′-CGACGCTAGCAGTGCTTGGTGGAG-3′ and 5′-TCTTGACAGCTTCACGAACTAAATTTT-3′ (24R) were used for the first-stage PCRs (an NheI site introduced by the codon alteration is underlined). The two first-stage PCR products were combined for the second stage and then amplified using primers 32F and 24R. For the conversion of codon 75 to proline, primers 32F and 5′-TCCACCAAGCACTGCAGGCGTCGCTATAATTC-3′ or 5′-GACGCCTGCAGTGCTTGGTGGAGCGGTTG-3′ and 24R were used for the first stage PCRs (a PstI site introduced by the codon alteration is underlined). For conversion of codons 79 and 80 from glycine to alanine and cysteine codons, respectively, primers 32F and 5′-GCTTGTTACAACCGCGCATGCAAGCACTGCTCCCG-3′ or 5′-CGGGAGCAGTGCTTGCATGCGCGGTTGTAACAAGC-3′ and 24R were used for the first-stage PCRs (an SphI site corresponding to the altered codons is underlined). For the simultaneous conversion of codons 75, 79, and 80 to leucine, alanine, and cysteine, respectively, primers 32F and 5′-GCATGCAAGCACTGCTAGCGTCGCTATAATTCCTTTC-3′ or 5′-CGCTAGCAGTGCTTGCATGCGCGGTTGTAACAAGC-3′ and 24R were used for the first-stage PCRs (NheI and SphI sites corresponding to the altered codons are underlined).
The 1.8-kb PCR products were digested with NsiI and HpaI and then used to replace the corresponding region of pB736flagR (this plasmid carries the entire gspB736flag coding sequence along with 1.2 kb of upstream DNA on the suicide vector pVA891 [9], and recombination within the upstream region facilitates the incorporation of the variant gspB sequences into the native chromosomal locus). Incorporation of only the intended codon substitutions was confirmed by DNA sequence analysis of the resulting plasmids, which were then used to transform PS846 or derivative strains as indicated.
Construction of gspB merodiploid strains.
Replacement of the three H-region glycine codons in the full-length gspB sequence was accomplished by transformation of M99 with pB736flagR*G3. To determine whether recombination had occurred downstream of the mutation (i.e., incorporating the mutation into the 5′ end of full-length gspB), transformants were examined by Southern blot analysis of chromosomal DNA that had been digested with SphI. The expression of GspB by one of the resulting strains (PS1144) was then compared with that of PS946. The latter strain was generated by transformation of M99 with pB736flagR, and therefore, both secrete GspB736flag and express native, cell surface GspB.
Construction of strains with multiple mutations.
Strains expressing variants of GspB in a secA2 mutant background were derived from PS516, which has an in-frame deletion within this gene (8). Strains expressing variants of GspB in a gtfA mutant background were, in some cases, derived from strains already carrying a nonpolar mutation in gtfA. Alternatively, a nonpolar gtfA mutation was introduced by replacement of the gene with a spectinomycin resistance cassette as described previously (50). Strains PS998 and PS999 were constructed by replacement of gspB in strains PS516 and PS727, respectively, using the plasmid pSgΔBR as described previously (9).
Transformation of S. gordonii.
DNA was introduced to M99 and derivative strains by natural transformation, as described previously (8). Gene replacement or plasmid integration at the expected site was confirmed by Southern blot analysis of chromosomal DNA extracted from the transformants.
Analysis of secreted, cell wall, and protoplast proteins.
Strains were grown for 18 h in THB containing erythromycin (15 μg/ml) and then diluted 10-fold into fresh THB and incubated for 6 h in the absence of erythromycin (the final cell density of all cultures was approximately 8 × 108 CFU per ml). For the analysis of secreted products, proteins were precipitated from culture supernatants by using trichloroacetic acid as described elsewhere (3). Cell wall proteins were extracted from S. gordonii strains by mutanolysin treatment (31). The mutanolysin extraction buffer included raffinose (26%, wt/vol) to maintain the integrity of the protoplasts. The secreted and cell wall proteins were combined with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and heated at 70°C for 10 min. For the analysis of cytoplasmic components, protoplasts (generated by digestion of the cell wall with mutanolysin) were lysed by suspension in SDS-PAGE sample buffer, followed by boiling for 10 min. The proteins present in 75 μl of spent culture medium (or in some cases, the proteins extracted from the walls of cells in 75 μl of culture) or the protoplasts of cells in 75 μl of culture were loaded for comparison of the relative amounts of protein present in the two fractions. A 50-kDa cytoplasmic protein that cross-reacts with the anti-FLAG antibody served as a loading control (not shown). The proteins were separated by SDS-PAGE through 3 to 8% Tris-acetate gels (Invitrogen) under reducing conditions. The proteins were transferred to BioTrace NT nitrocellulose membranes (Pall Corporation) using an XCell SureLock transfer apparatus (Invitrogen). For Western blot analysis, the membranes were incubated for 1 h in a suspension of 1× blocking reagent (Roche) in DPBS. An anti-FLAG monoclonal antibody was used at a concentration of 0.5 μg/ml, and peroxidase-conjugated anti-mouse antibodies were used at a 1:10,000 dilution. For the analysis of native GspB expression by the merodiploid strains, a goat polyclonal anti-GspB serum (8) was used at a 1:5,000 dilution, along with peroxidase-conjugated anti-goat antibodies at a 1:25,000 dilution. Glycosylated variants of GspB were detected by lectin blot analysis (a highly sensitive means of detecting the presence of carbohydrate on GspB), using biotinylated sWGA and peroxidase-conjugated streptavidin as described previously (49). All blots were developed with the SuperSignal West Pico chemiluminescent substrate (Pierce). Where indicated, signal intensities were quantified using ImageQuant version 5.2 software (Molecular Dynamics), normalized to the intensity of the 50-kDa loading control, and then compared.
Effect of sodium azide on nascent protein expression.
The inhibition of SecA-dependent protein transport was performed as described by Jongbloed et al. (25), with modifications. Strains were grown for 18 h in 3 ml THB. After centrifugation at 3,200 × g for 10 min, the cells were suspended in 3 ml fresh medium, split into three 1-ml portions, and then either left untreated or combined with 2 or 6 μl of 5% sodium azide. The cultures were incubated at 37°C for 90 min and then the medium and protoplast fractions were prepared and analyzed as described above.
Secondary structure prediction and hydropathy analysis.
The effect of glycine substitutions in the GspB signal sequence on α-helix formation was determined using the Chou and Fasman method of secondary structure prediction (13). Changes in the hydrophobicity of the signal sequences were determined by the method of Kyte and Doolittle (27).
RESULTS
Conserved motifs in the amino termini of GspB homologues.
A number of serine-rich glycoprotein family members have been characterized (21, 35, 40, 42, 44, 56) or identified through analysis of the available genome sequence data (50). Although each protein has a C-terminal cell wall sorting signal, most are not predicted to be secreted, as determined by using the SignalP 3.0 algorithm (5; data not shown). As one approach to identifying components of the GspB signal sequence that are critical for routing preproteins toward the accessory Sec components, the N-terminal ends of GspB and seven homologues were compared. Upon alignment, two conserved elements are evident: a KSGKXW motif in the N region and two or three glycine residues in the H region (Fig. 2B). In a previous report, we examined the effect on export of deleting 60 residues, including the KSGKXW motif, from within the N region (9). As noted above, the results in that study indicated that the extended N region was important for the translocation of variants of GspB via both the accessory and canonical Sec pathways. The impact of glycine residues in the H region on export, however, had not been addressed.
Replacement of glycine residues in the signal sequence hydrophobic core enables SecA2-independent export of GspB736flag.
Residues in the H domain of signal peptides have been shown to adopt an α-helical conformation in the membrane (11), and early experiments by Emr and Silhavy (19) indicated that α-helix formation is essential for correct functioning of the Escherichia coli LamB signal sequence. A recent report by Adams et al. (1) demonstrated that a glycine residue within the H region (at −10 relative to the signal peptidase cleavage site) was important for routing export towards the SecB versus the SRP (signal recognition particle) pathway in E. coli. That is, replacement of the glycine with residues that promote α-helix formation led to enhanced interaction of the preproteins with the SRP. Based in part on these findings, we hypothesized that glycine residues in the hydrophobic core of the GspB signal sequence might play an analogous role in trafficking of the preprotein in S. gordonii.
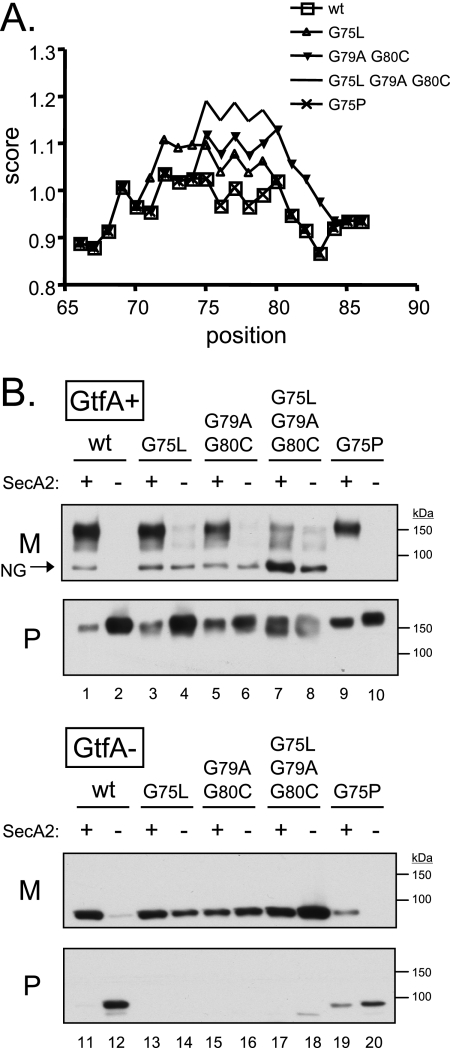
To determine whether helix-breaking residues in the H region of the GspB signal sequence are critical for export, each of the glycines was changed to a residue expected to promote α-helix formation (alanine, cysteine, or leucine) (Fig. 3A). As shown previously (9), the export of GspB736flag from a GtfA+ strain is dependent on SecA2, in that disruption of secA2 results in a loss of GspB736flag secretion into the culture medium and retention of the protein in the cytoplasm (Fig. 3B, upper panel, lanes 1 and 2). Additionally, a relatively minor amount of nonglycosylated GspB736flag, as indicated by the apparent molecular mass and a failure to react with sWGA (data not shown), could be detected in the culture medium (Fig. 3B, upper panel, arrow). Conversion of codon 75 from glycine to leucine had no noticeable effect on GspB736flag export in a wild-type background (Fig. 3B, upper panel, lane 3). However, when expressed in a secA2 mutant background (Fig. 3B, upper panel, lane 4), export of the G75L variant was severely reduced but, surprisingly, not abolished. Conversion of glycine codons 79 and 80 to alanine and cysteine, respectively, led to a slight reduction in the export of GspB736flag (27% decrease in the medium fraction and 138% increase in the protoplast fraction, as measured by densitometry) (Fig. 3B, upper panel, lane 5 versus lane 1). When the G79A/G80C variant was expressed in a secA2 mutant background (Fig. 3B, upper panel, lane 6), export was again severely reduced but not abolished. A variant of GspB736flag in which all three of the glycine residues in the H region were changed to helix-promoting residues was also generated (GspB736flag*G3). When compared with the results of conversion of just G75 or the G79/G80 pair, the triple glycine substitution resulted in export that was even less dependent on SecA2 (Fig. 3B, upper panel, lanes 7 and 8). Moreover, the glycoprotein migrated as a doublet rather than a broad band, suggesting that it may be differently glycosylated or differently processed. Furthermore, the nonglycosylated form of GspB736flag was much more readily detected. These results suggest that the H-region glycine residues render the preprotein unsuitable for export by means other than the accessory Sec pathway and that alterations in the hydrophobic core concomitantly lead to alterations in the extent of glycosylation of the secreted protein.
FIG. 3.
(A) Predicted effect of amino acid substitutions on alpha-helix formation within the H region of the GspB signal sequence (positions 65 through 86). Data were obtained from the ExPASy ProtScale analysis website (http://www.expasy.org/tools/protscale.html), using the Chou and Fasman scale (13). (B) Export of GspB736flag variants carrying substitutions in the H region of the signal peptide. Proteins were detected by using an anti-FLAG monoclonal antibody. The lanes contain proteins present in 75 μl of cultures grown for 6 h. The arrow indicates a nonglycosylated (NG) form of GspB736flag secreted by the GtfA+ strains (determined by migration under SDS-PAGE with an apparent molecular mass that is equivalent to the predicted mass of 80 kDa and by failure to react with sWGA; data not shown). M, medium fraction; P, protoplast fraction.
Previous studies indicated that carbohydrate moieties on GspB can interfere with export via the canonical Sec pathway (9). To more clearly examine the routing of the GspB736flag polypeptide, we also examined the effect of the glycine substitutions on the export of GspB variants in the absence of glycosylation, thus eliminating any inhibition of export that may be due to carbohydrate moieties. As described above, when expressed in a gtfA mutant background (which results in complete abolition of glycosylation [49]), GspB736flag can be inefficiently exported by the canonical Sec system (Fig. 3B, lower panel, lanes 11 and 12). In contrast to the results seen with the GtfA+ strains, each of the glycine-substituted variants was efficiently secreted by the gtfA mutant strains, with little or no preprotein detected in the protoplasts (Fig. 3B, lower panel, lanes 13, 15, and 17). In addition, the nonglycosylated variants were more readily exported in a SecA2-independent manner (Fig. 3B, lower panel, lanes 14, 16, and 18). The combined results confirm that glycine residues in the hydrophobic core of the signal sequence facilitate the export of GspB736flag via the accessory Sec pathway and prevent export via an alternate route.
Proline cannot substitute for glycine.
To determine whether disruption of α-helix formation is the sole function of the glycine residues in the GspB signal sequence, we next examined whether an alternate helix-breaking residue could substitute for glycine. Replacement of G75 with proline led to decreased export via the accessory Sec pathway from both GtfA+ and GtfA− strains (Fig. 3B, lanes 9 and 19, respectively) and abolished SecA2-independent export (lanes 10 and 20). The results indicate that an alternate helix-breaking residue in the H region of the signal sequence cannot substitute for glycine and can hinder export via both the accessory Sec pathway and the alternate route.
The H-region glycine residues are essential for export of native GspB.
In order to determine whether the H-region glycine residues were important for the export of full-length GspB, the triple Gly change was introduced into the native GspB sequence. Plasmid pB736flagR*G3 was crossed into the M99 chromosome such that the resulting merodiploid strains express both GspB736flag and GspB, one of which has a native signal sequence and one of which has the triple Gly mutation. As seen in Fig. 4, the replacement of glycine residues in the H region led to severely impaired export of native GspB. These results indicate that glycine residues in the hydrophobic core of GspB signal sequence are essential for the translocation of native GspB and that, although some truncated, glycosylated forms of GspB can be exported via an alternate pathway (Fig. 3B), the full-length glycoprotein cannot.
FIG. 4.
(A) Construction of the merodiploid strain PS1144. The dashed line in the PS1144 chromosomal locus represents the plasmid backbone (not drawn to scale). (B) Export of GspB and GspB736flag by merodiploid strains. Lane 1, PS946 (gspB736flag gspB); lane 2, PS1144 (gspB736flag gspB*G3). The lanes contain proteins present in 75 μl of cultures grown for 6 h. Proteins present in the protoplast (P), cell wall (W), or culture medium (M) were detected by Western blotting with the indicated antibodies.
Glycine residues in the H region inhibit export via the canonical Sec pathway.
The ATPase activity of SecA is highly sensitive to sodium azide, and exposure of cells to azide is an established means to inhibit Sec-dependent protein transport. In order to assess whether export of the H-region variants was occurring via the canonical Sec pathway, export of GspB736flag*G3 from the secA2 mutant strain PS1146 in the presence and absence of sodium azide was examined. Exposure of cells to as little as 1.5 mM azide led to a severe reduction in GspB736flag*G3 export, and 4.5 mM azide nearly abolished export (Fig. 5). This strongly suggests that translocation of GspB736flag*G3 occurs via the canonical Sec pathway.
FIG. 5.
Effect of sodium azide on export of GspB736flag*G3 from secA2 mutant strain PS1146. The lanes contain protein from 75 μl of cultures grown at high density for 90 min. Proteins were detected by using an anti-FLAG monoclonal antibody. M, medium fraction; P, protoplast fraction.
The H region, and not the N region, inhibits export via the canonical Sec pathway.
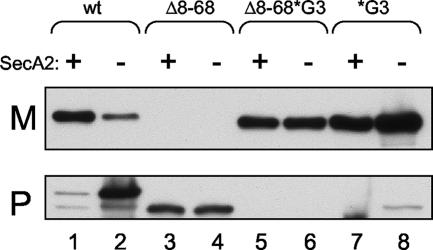
As noted above, we previously examined the effect of deleting residues G8 through L68 of the extended N region of the GspB signal sequence (9). As reported in that study, although the resulting N terminus was predicted to function as a canonical signal sequence, the GspB736flagΔ8-68 variant was not exported via either the canonical or accessory Sec pathways, even when expressed in a gtfA mutant background (results are shown here in Fig. 6, lanes 3 and 4). Based on our new findings, we now asked whether the H region of GspB736flagΔ8-68 inhibited export via the canonical Sec pathway. A variant of GspB736flag that has both the N-region deletion and the H-region glycine substitutions (GspB736flagΔ8-68*G3) was generated and was found to be readily exported (Fig. 6, lane 5). Moreover, export of this variant appeared to be independent of the accessory Sec system (Fig. 6, lane 6). However, the yield of exported protein was somewhat less than that of the GspB736flag*G3 variant, which has the intact N region (Fig. 6, lanes 7 and 8). The results indicate that the main reason the GspB signal sequence is not efficiently recognized by the canonical Sec system is because of glycine residues in the H region, not because of interference by the N region.
FIG. 6.
Effect of mutations in the signal sequence on export of nonglycosylated GspB736flag variants from gtfA mutant strains. Proteins were detected by using an anti-FLAG monoclonal antibody. The lanes contain proteins present in 75 μl of cultures grown for 6 h. M, medium fraction; P, protoplast fraction. Lane 1, PS1179; lane 2, PS1177; lane 3, PS1033; lane 4, PS1172; lane 5, PS1171; lane 6, PS1139; lane 7, PS1136; lane 8, PS1137.
DISCUSSION
Previous studies with S. gordonii have demonstrated that the accessory Sec system is essential for the export of native (i.e., full-length and glycosylated) GspB. There are at least two key aspects of GspB structure that affect transport. First, the polypeptide backbone can only be inefficiently translocated by the canonical Sec system. Second, the carbohydrate moieties of GspB, which are essential for preprotein stability, further hinder export via that route. The experiments presented in this report demonstrate that the H region of the GspB signal sequence is an important feature contributing to the specialized export of this unusual adhesin and, along with the carbohydrate, is a primary deterrent to translocation by the canonical Sec system. Thus, these studies provide a more complete picture of the means by which GspB is selectively transported by the accessory Sec system.
The data presented here highlight the relative importance of the N and H regions of the GspB signal peptide for trafficking of the preprotein. Whereas the atypically long N region is compatible with export via both the canonical Sec and accessory Sec pathways, the H region appears to be specifically tailored for the accessory Sec system. Replacement of just one of three glycine residues in the H region (G75) clearly had an effect on trafficking, since the preprotein could be translocated more readily in a SecA2-independent manner. Replacement of the G79/G80 pair similarly enhanced SecA2-independent transport and simultaneously led to a decrease in the efficiency of SecA2-dependent transport. Replacement of all three glycine residues resulted in a more severe loss of export via the accessory Sec pathway, as evidenced by the SecA2-independent secretion of GspB736flag*G3 and by the failure of full-length GspB*G3 to be exported. Thus, glycine residues in the hydrophobic core of the signal sequence not only are essential for export via the accessory Sec pathway but also hinder export via the canonical Sec route. Subtle variations in the H region (one or two glycine substitutions) have a more pronounced effect in the GtfA+ strains, most likely due to a negative effect of the carbohydrate moieties on the rate of export via the canonical Sec system. Moreover, although some glycosylated forms of GspB736flag can be translocated by the canonical Sec route, full-length GspB cannot. This may be due either to the additional bulk of carbohydrate on the full-length glycoprotein or to the conformation of the preprotein or both (note that nonglycosylated full-length GspB is highly unstable and tends to aggregate, so any translocation of unglycosylated GspB*G3 that might have occurred via the canonical Sec pathway would not have been detected).
As yet, the means by which the glycine residues interfere with translocation by the canonical Sec system are not clearly defined. Intuitively, the GspB signal sequence must necessarily prevent cotranslational export, since this would preclude cytoplasmic glycosylation. Replacement of the glycine residues in the GspB signal sequence not only led to an increase in the predicted α-helicity of the H region (Fig. 3A) but also resulted in a subtle increase in hydrophobicity (data not shown). Studies with E. coli indicate that the hydrophobicity of the H region can affect routing towards particular export pathways. For example, a linear correlation has been noted between signal sequence hydrophobicity and cross-linking to the SRP (53). Thus, increasing the hydrophobicity of some signal sequences can reroute SecB-dependent proteins to the SRP pathway (10, 29). In addition, the signal sequences of proteins destined for export via the TAT pathway tend to be less hydrophobic than those exported by the Sec pathway (16). Far less is known regarding the roles of signal sequences in protein trafficking in gram-positive bacteria. The signal peptides of gram-positive organisms are overall slightly more hydrophobic than those of gram-negative organisms (55), and this has been proposed to facilitate SRP-dependent export (52). Although studies with Bacillus subtilis have shown that most of the proteins secreted by this organism are dependent on the SRP (23, 58), the SRP is not essential for viability (under nonstress conditions) or for the translocation of most proteins in Streptococcus mutans (17, 22). It is therefore unclear whether gram-positive bacteria, which lack an apparent SecB homologue, have distinctly different pathways that shuttle preproteins to the SecYEG translocase and whether interference with SRP binding would be central to the trafficking of GspB.
Although the H-region glycine residues may constitute a novel type of avoidance motif for the canonical Sec system, it is also possible that the glycine residues might have an effect on structure or hydrophobicity that is optimal for the interaction of the preprotein with the accessory Sec components. This is suggested by two key findings. First, mutation of the G79/G80 pair in a GtfA+ background resulted in decreased export of GspB736flag via the accessory Sec pathway (Fig. 3B, lane 5). Second, upon alteration of the H region, full-length GspB accumulated in the cytoplasm (Fig. 4B). Several reports have suggested that helix-breaking residues in the hydrophobic core of signal sequences may affect the formation of a hairpin that facilitates membrane insertion (14, 18, 36). However, a helix-breaking proline residue cannot substitute for glycine in the GspB signal sequence, which suggests that the glycine residues may function other than to disrupt a helical structure. Glycine residues are prevalent in the transmembrane helices of membrane proteins and have been shown to facilitate helix packing between the transmembrane domains of polytopic membrane proteins (24, 28). Moreover, a GXXXG motif occurs frequently at helix-helix interfaces in both soluble and membrane proteins and is essential for the dimerization of a variety of single-pass membrane proteins (2, 26, 37, 41). Thus, it is possible that the H-region glycine residues are necessary to facilitate protein-protein interactions between the GspB preprotein and components of the accessory Sec translocase. The hydrophobic core of canonical signal peptides intercalates between Tm2b and Tm7 of SecY (33), and it is reasonable to speculate that the GspB signal sequence interacts with the corresponding transmembrane domains of SecY2. It may be noteworthy that the streptococcal homologues of GspB have a GAXXG motif centered in the H region, whereas the staphylococcal homologues have a GG motif, and it will be interesting to determine whether this reflects a difference in the mechanisms of interaction of the respective preproteins with the accessory Sec translocase in these two genera.
The results presented here also confirm that the extended N region is critical for the export of GspB variants, as long as the native H region is present. We have identified a KSGKXW motif within the N region that is conserved among the family of GspB homologues. This is somewhat similar to the YSIRK motif in S. aureus signal sequences characterized by Bae and Schneewind (4), which affects signal peptide processing but is not essential for translocation. A similar (YSLRK) motif in the signal sequence of the Streptococcus pyogenes M protein is also not essential for transport (12); therefore, the reason for the apparent conservation of these motifs remains unclear. It is possible that the N region of the GspB signal sequence helps to maintain the preprotein in an export-competent state, and indeed, a known function of signal peptides is to retard the folding of the mature domain of preproteins (34). The atypically long N regions of proteins transported by the accessory Sec route may be ideally matched to facilitate export of the serine-rich proteins, and previous studies indicated that the GspB signal sequence does not readily support the translocation of heterologous proteins (9). It is additionally possible that the extended N region can interact with components of both export pathways but that the structure of the H region somehow greatly favors interaction with the accessory Sec system. Future studies will examine the extent to which the nascent preproteins utilized here can interact with SecA2 versus SecA, with the SRP, and with the accessory and canonical Sec translocases.
Acknowledgments
This work was supported by the Department of Veterans Affairs and by grants RO1AI41513 and RO1AI057433 from the National Institutes of Health.
Footnotes
Published ahead of print on 16 March 2007.
REFERENCES
- 1.Adams, H., P. A. Scotti, H. De Cock, J. Luirink, and J. Tommassen. 2002. The presence of a helix breaker in the hydrophobic core of signal sequences of secretory proteins prevents recognition by the signal-recognition particle in Escherichia coli. Eur. J. Biochem. 269:5564-5571. [DOI] [PubMed] [Google Scholar]
- 2.Arbely, E., Z. Granot, I. Kass, J. Orly, and I. T. Arkin. 2006. A trimerizing GxxxG motif is uniquely inserted in the severe acute respiratory syndrome (SARS) coronavirus spike protein transmembrane domain. Biochemistry 45:11349-11356. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology. John Wiley & Sons, New York, NY.
- 4.Bae, T., and O. Schneewind. 2003. The YSIRK-G/S motif of staphylococcal protein A and its role in efficiency of signal peptide processing. J. Bacteriol. 185:2910-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 6.Bensing, B. A., B. W. Gibson, and P. M. Sullam. 2004. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J. Bacteriol. 186:638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bensing, B. A., J. A. Lopez, and P. M. Sullam. 2004. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect. Immun. 72:6528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081-1094. [DOI] [PubMed] [Google Scholar]
- 9.Bensing, B. A., D. Takamatsu, and P. M. Sullam. 2005. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 58:1468-1481. [DOI] [PubMed] [Google Scholar]
- 10.Bowers, C. W., F. Lau, and T. J. Silhavy. 2003. Secretion of LamB-LacZ by the signal recognition particle pathway of Escherichia coli. J. Bacteriol. 185:5697-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briggs, M. S., D. G. Cornell, R. A. Dluhy, and L. M. Gierasch. 1986. Conformations of signal peptides induced by lipids suggest initial steps in protein export. Science 233:206-208. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson, F., M. Stalhammar-Carlemalm, K. Flardh, C. Sandin, E. Carlemalm, and G. Lindahl. 2006. Signal sequence directs localized secretion of bacterial surface proteins. Nature 442:943-946. [DOI] [PubMed] [Google Scholar]
- 13.Chou, P. Y., and G. D. Fasman. 1978. Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. Relat. Areas Mol. Biol. 47:45-148. [DOI] [PubMed] [Google Scholar]
- 14.Chupin, V., J. A. Killian, J. Breg, H. H. de Jongh, R. Boelens, R. Kaptein, and B. de Kruijff. 1995. PhoE signal peptide inserts into micelles as a dynamic helix-break-helix structure, which is modulated by the environment. A two-dimensional 1H NMR study. Biochemistry 34:11617-11624. [DOI] [PubMed] [Google Scholar]
- 15.Claverys, J. P., A. Dintilhac, E. V. Pestova, B. Martin, and D. A. Morrison. 1995. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164:123-128. [DOI] [PubMed] [Google Scholar]
- 16.Cristobal, S., J. W. de Gier, H. Nielsen, and G. von Heijne. 1999. Competition between Sec- and TAT-dependent protein translocation in Escherichia coli. EMBO J. 18:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowley, P. J., G. Svensater, J. L. Snoep, A. S. Bleiweis, and L. J. Brady. 2004. An ffh mutant of Streptococcus mutans is viable and able to physiologically adapt to low pH in continuous culture. FEMS Microbiol. Lett. 234:315-324. [DOI] [PubMed] [Google Scholar]
- 18.de Vrije, G. J., A. M. Batenburg, J. A. Killian, and B. de Kruijff. 1990. Lipid involvement in protein translocation in Escherichia coli. Mol. Microbiol. 4:143-150. [DOI] [PubMed] [Google Scholar]
- 19.Emr, S. D., and T. J. Silhavy. 1983. Importance of secondary structure in the signal sequence for protein secretion. Proc. Natl. Acad. Sci. USA 80:4599-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 4:1603-1605. [DOI] [PubMed] [Google Scholar]
- 21.Handley, P. S., F. F. Correia, K. Russell, B. Rosan, and J. M. DiRienzo. 2005. Association of a novel high molecular weight, serine-rich protein (SrpA) with fibril-mediated adhesion of the oral biofilm bacterium Streptococcus cristatus. Oral Microbiol. Immunol. 20:131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasona, A., K. Zuobi-Hasona, P. J. Crowley, J. Abranches, M. A. Ruelf, A. S. Bleiweis, and L. J. Brady. 2007. Membrane composition changes and physiological adaptation by Streptococcus mutans signal recognition particle pathway mutants. J. Bacteriol. 189:1219-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirose, I., K. Sano, I. Shioda, M. Kumano, K. Nakamura, and K. Yamane. 2000. Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology 146:65-75. [DOI] [PubMed] [Google Scholar]
- 24.Javadpour, M. M., M. Eilers, M. Groesbeek, and S. O. Smith. 1999. Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys. J. 77:1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jongbloed, J. D., H. Antelmann, M. Hecker, R. Nijland, S. Bron, U. Airaksinen, F. Pries, W. J. Quax, J. M. van Dijl, and P. G. Braun. 2002. Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis. J. Biol. Chem. 277:44068-44078. [DOI] [PubMed] [Google Scholar]
- 26.Kleiger, G., R. Grothe, P. Mallick, and D. Eisenberg. 2002. GXXXG and AXXXA: common alpha-helical interaction motifs in proteins, particularly in extremophiles. Biochemistry 41:5990-5997. [DOI] [PubMed] [Google Scholar]
- 27.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 28.Landolt-Marticorena, C., K. A. Williams, C. M. Deber, and R. A. Reithmeier. 1993. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol. 229:602-608. [DOI] [PubMed] [Google Scholar]
- 29.Lee, H. C., and H. D. Bernstein. 2001. The targeting pathway of Escherichia coli presecretory and integral membrane proteins is specified by the hydrophobicity of the targeting signal. Proc. Natl. Acad. Sci. USA 98:3471-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macrina, F. L., R. P. Evans, J. A. Tobian, D. L. Hartley, D. B. Clewell, and K. R. Jones. 1983. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene 25:145-150. [DOI] [PubMed] [Google Scholar]
- 31.McNab, R., and H. F. Jenkinson. 1998. Lipoproteins and other cell-surface associated proteins in streptococci. Methods Cell Sci. 20:209-216. [Google Scholar]
- 32.Obert, C., J. Sublett, D. Kaushal, E. Hinojosa, T. Barton, E. I. Tuomanen, and C. J. Orihuela. 2006. Identification of a candidate Streptococcus pneumoniae core genome and regions of diversity correlated with invasive pneumococcal disease. Infect. Immun. 74:4766-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osborne, A. R., T. A. Rapoport, and B. van den Berg. 2005. Protein translocation by the Sec61/SecY channel. Annu. Rev. Cell Dev. Biol. 21:529-550. [DOI] [PubMed] [Google Scholar]
- 34.Park, S., G. Liu, T. B. Topping, W. H. Cover, and L. L. Randall. 1988. Modulation of folding pathways of exported proteins by the leader sequence. Science 239:1033-1035. [DOI] [PubMed] [Google Scholar]
- 35.Plummer, C., H. Wu, S. W. Kerrigan, G. Meade, D. Cox, and C. W. I. Douglas. 2005. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br. J. Haematol. 129:101-109. [DOI] [PubMed] [Google Scholar]
- 36.Rizo, J., F. J. Blanco, B. Kobe, M. D. Bruch, and L. M. Gierasch. 1993. Conformational behavior of Escherichia coli OmpA signal peptides in membrane mimetic environments. Biochemistry 32:4881-4894. [DOI] [PubMed] [Google Scholar]
- 37.Russ, W. P., and D. M. Engelman. 2000. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 296:911-919. [DOI] [PubMed] [Google Scholar]
- 38.Schneewind, O., D. Mihaylova-Petkov, and P. Model. 1993. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 12:4803-4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneewind, O., P. Model, and V. A. Fischetti. 1992. Sorting of protein A to the staphylococcal cell wall. Cell 70:267-281. [DOI] [PubMed] [Google Scholar]
- 40.Seifert, K. N., E. E. Adderson, A. A. Whiting, J. F. Bohnsack, P. J. Crowley, and L. J. Brady. 2006. A unique serine-rich repeat protein (Srr-2) and novel surface antigen (epsilon) associated with a virulent lineage of serotype III Streptococcus agalactiae. Microbiology 152:1029-1040. [DOI] [PubMed] [Google Scholar]
- 41.Senes, A., M. Gerstein, and D. M. Engelman. 2000. Statistical analysis of amino acid patterns in transmembrane helices: the GxxxG motif occurs frequently and in association with beta-branched residues at neighboring positions. J. Mol. Biol. 296:921-936. [DOI] [PubMed] [Google Scholar]
- 42.Siboo, I. R., H. F. Chambers, and P. M. Sullam. 2005. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect. Immun. 73:2273-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullam, P. M., F. H. Valone, and J. Mills. 1987. Mechanisms of platelet aggregation by viridans group streptococci. Infect. Immun. 55:1743-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi, Y., K. Konishi, J. O. Cisar, and M. Yoshikawa. 2002. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infect. Immun. 70:1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi, Y., A. L. Sandberg, S. Ruhl, J. Muller, and J. O. Cisar. 1997. A specific cell surface antigen of Streptococcus gordonii is associated with bacterial hemagglutination and adhesion to α2-3-linked sialic acid-containing receptors. Infect. Immun. 65:5042-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi, Y., E. Takashima, K. Shimazu, H. Yagishita, T. Aoba, and K. Konishi. 2006. Contribution of sialic acid-binding adhesin to pathogenesis of experimental endocarditis caused by Streptococcus gordonii DL1. Infect. Immun. 74:740-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takamatsu, D., B. A. Bensing, H. Cheng, G. A. Jarvis, I. R. Siboo, J. A. Lopez, J. M. Griffiss, and P. M. Sullam. 2005. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibα. Mol. Microbiol. 58:380-392. [DOI] [PubMed] [Google Scholar]
- 48.Takamatsu, D., B. A. Bensing, A. Prakobphol, S. J. Fisher, and P. M. Sullam. 2006. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infect. Immun. 74:1933-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2004. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J. Bacteriol. 186:7100-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2004. Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol. Microbiol. 52:189-203. [DOI] [PubMed] [Google Scholar]
- 51.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2005. Two additional components of the accessory Sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J. Bacteriol. 187:3878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tjalsma, H., A. Bolhuis, J. D. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valent, Q. A., J. W. de Gier, G. von Heijne, D. A. Kendall, C. M. ten Hagen-Jongman, B. Oudega, and J. Luirink. 1997. Nascent membrane and presecretory proteins synthesized in Escherichia coli associate with signal recognition particle and trigger factor. Mol. Microbiol. 25:53-64. [DOI] [PubMed] [Google Scholar]
- 54.von Heijne, G. 1990. The signal peptide. J. Membr. Biol. 115:195-201. [DOI] [PubMed] [Google Scholar]
- 55.von Heijne, G., and L. Abrahmsen. 1989. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 244:439-446. [DOI] [PubMed] [Google Scholar]
- 56.Wu, H., and P. M. Fives-Taylor. 1999. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol. Microbiol. 34:1070-1081. [DOI] [PubMed] [Google Scholar]
- 57.Yajima, A., Y. Takahashi, and K. Konishi. 2005. Identification of platelet receptors for the Streptococcus gordonii DL1 sialic acid-binding adhesin. Microbiol. Immunol. 49:795-800. [DOI] [PubMed] [Google Scholar]
- 58.Zanen, G., H. Antelmann, R. Meima, J. D. Jongbloed, M. Kolkman, M. Hecker, J. M. van Dijl, and W. J. Quax. 2006. Proteomic dissection of potential signal recognition particle dependence in protein secretion by Bacillus subtilis. Proteomics 6:3636-3648. [DOI] [PubMed] [Google Scholar]